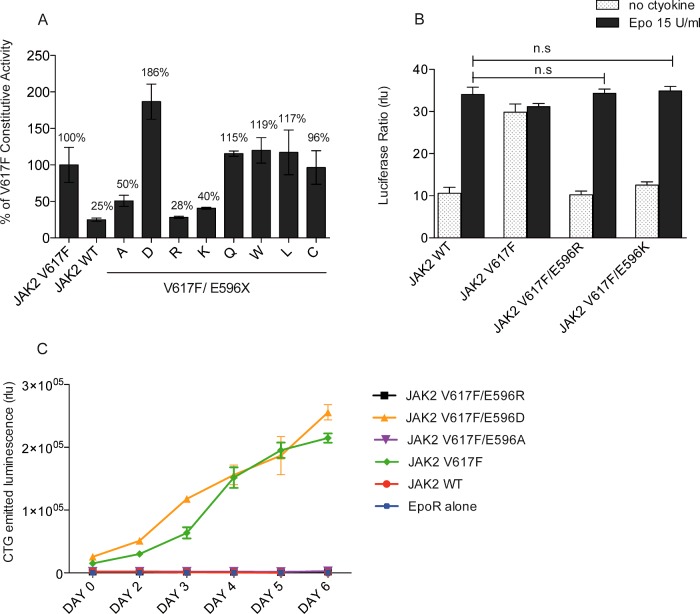
Figure 2. Investigation of the nature of the interaction mediated by E596 in V617F activation.
(A) STAT5 transcriptional activity was measured in γ2A cells by luciferase assay. Mutation of E596 to either arginine or lysine induces a decrease in constitutive activity of JAK2 V617F up to 71% and 60% respectively whereas substitution to aspartate increases the activity of the mutant protein up to 86%. The activity of the wild-type JAK2 amounted to 25% of the activity of JAK2 V617F. Shown are means for three independent experiments each performed in triplicate and data were normalized by taking 100% as the basal activity of JAK2 V617F. (B) Substitution of E596 by protonated residues in JAK2 V617F displays an Epo-induced response similar to JAK2 WT. These two mutants behave like the wild-type JAK2. Shown are means±S.E.M. for three independent experiments done in triplicate. n.s, not significant. (C) BaF3-EpoR stably expressing JAK2 double mutants were starved for 4 h and allow to grow in a cytokine-free media. The number of living cells was evaluated by ATP measurement during 6 days. Only JAK2 V617F and JAK2 V617F/E596D transform the BaF3 into factor-independent cells. Although E596/K and A mutations prevent autonomous grow of JAK2 V617F.