Fig. 5.
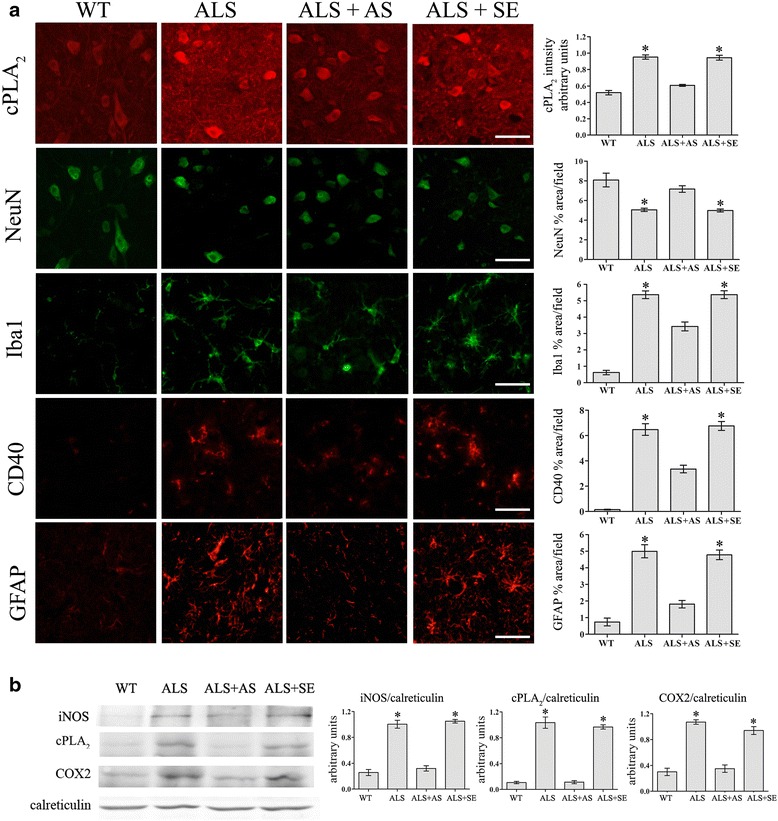
Reduction of cPLA2α upregulation in the spinal cord of hmSOD1 mice shortly before the onset of motor dysfunction reduced neuronal death and gliosis. Fifteen-week-old hmSOD1 mice were brain infused with 10 μg/day AS or the corresponding sense for 3–4 weeks and analyzed at week 18–19. a Representative immunofluorescence staining of cPLA2α, neurons (NeuN), microglia (Iba-1), and astrocytes (GFAP) in the spinal cord tissue section of WT mice, hmSOD1 mice (ALS), hmSOD1 mice brain infused with AS (ALS + AS) or sense (ALS + SE) at 18–19 weeks. Scale bars = 50 μm. Shown are representative images of 12 mice in each group. The mean ± SEM of the fluorescence intensity (for cPLA2α) and percentage of the cell area (for cell markers) are presented in the bar graph. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice. b Representative immunoblot analysis of cPLA2α, COX-2, and iNOS and the corresponding calreticulin protein expression in the spinal cord lysates of the mice. Densitometry analysis for the three proteins was done as described for cPLA2α in Fig. 3b. The bar graph is the mean ± SE of 8 mice in each group. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice
