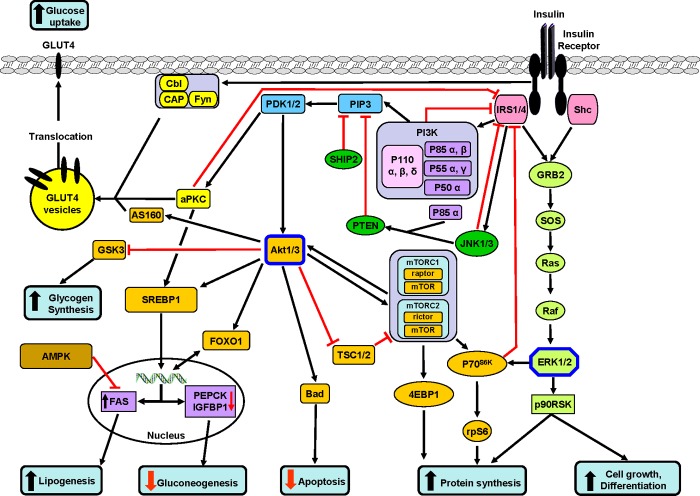
Figure 1. Following insulin binding, the IR auto-phosphorylates and then phosphorylates its substrates including the IRSs and Shc.
Following phosphorylation, specific domains in these substrates interact with other proteins. One of the major complexes interacting with IRSs is PI3K, which is formed of two interacting proteins: a regulatory subunit (p85) and a catalytic subunit (p110). There are several isoforms of both subunits: p85α, p55α, p50α, p85β and p55γ for the regulatory subunit and p110α, β and δ for the catalytic subunit. The binding of the regulatory subunit to IRS forms an active heterodimer which phosphorylates membrane phosphatidylinositol residues in the 3′ position of PIP3 to form PDK1/2 (shown in blue). In turn, PDK1 phosphorylates (on threonine and serine residues) and thereby activates Akt/PKB (a serine/threonine kinase, with three isoforms). Downstream (orange boxes), Akt phosphorylates several kinases or transcription factors (some examples are shown) involved in most of the major metabolic effects of insulin: (i) glycogen synthesis through GSK3, which is inactivated by phosphorylation. The inactivation of GSK3 leads to the de-phosphorylation and activation of GS and hence to an acceleration of glycogen synthesis, (ii) inhibition of gluconeogenesis by turning off the PEPCK gene through inhibition of FOXO1 function (mainly in liver and adipose tissue), (iii) protein synthesis through mTOR and subsequent activation of p70S6K and 4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1), (iv) the Akt pathway also stimulates cell growth and differentiation (not shown in the figure) and (v) exerts anti-apoptotic effects by phosphorylating the anti-apoptotic protein, Bad. The kinase, PI3K also activates aPKCs (atypical PKCs) (yellow boxes), which in concert with Akt (via AS160) exerts different effects according to the tissue: in muscle and adipocytes they initiate the translocation of intracellular GLUT4 on to the membrane and thereby stimulate glucose transport into the cell. Another important and well-documented signalling pathway is the Ras/ERK pathway (green boxes). The binding of Grb2 (growth factor receptor-bound protein 2) and Sos (son of sevenless) to tyrosine-phosphorylated IRS or Shc can also activate the small GTPase Ras cascade, Raf and ultimately ERK1 and ERK2 (green boxes). When activated, these serine kinases simulate protein synthesis through the activation of p90S6K and activate transcription factors and ultimately cell multiplication and differentiation. Some lipid phosphatases including PTEN and SHIP2 act on PIP3 to decrease or extinguish the insulin signal. Some of the insulin-stimulated serine kinases (aPKC, JNKs, p90RSK, ERKs and S6 kinase) may also exert negative effects on insulin sensitivity. Stimulatory connections are shown in black and inhibitory ones are shown in red.