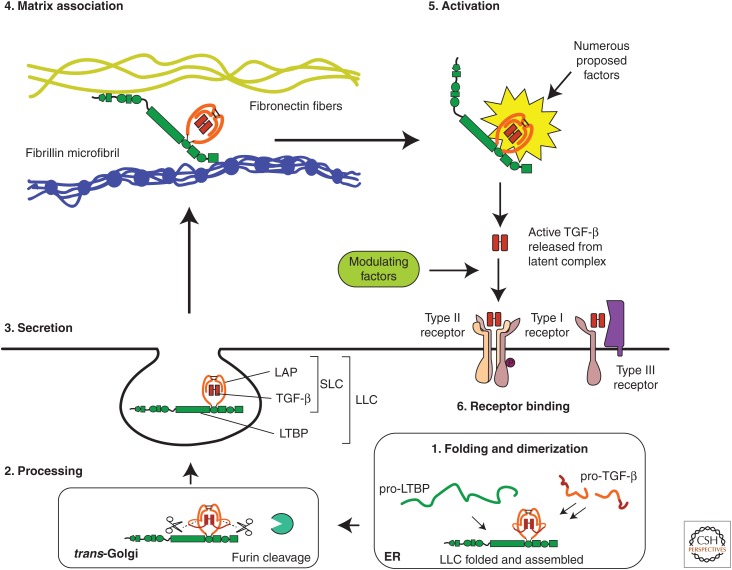
Figure 1.
Scheme for the secretion and extracellular regulation of transforming growth factor β (TGF-β). Starting at the bottom right (1), TGF-β and latent TGF-β-binding protein (LTBP) are translated into the endoplasmic reticulum (ER) where pro-TGF-β dimerizes and is then disulfide bonded to LTBP to form a ternary complex. The TGF-β dimer is cleaved from its pro-peptide (latency-associated peptide [LAP]) in the trans-Golgi network, but TGF-β and LAP remain strongly associated via noncovalent interactions forming the large latent complex (LLC) (lower left) (2). Once secreted (middle left) (3), the LTBP may bind various matrix fibers that sequester latent TGF-β until it is released by an activator (upper left) (4). The latent complex is then activated (upper right) (5), by one of several potential mechanisms, releasing the mature TGF-β. Active TGF-β may bind to cell-surface receptors (lower right) (6), although other factors may also bind the active growth factor at this stage and either inhibit or promote receptor binding.