Figure 1. Hyperglycemia-induced myocardial protein modification by O-GlcNAc causes increased intracellular Ca++ and delayed afterpolarizations.
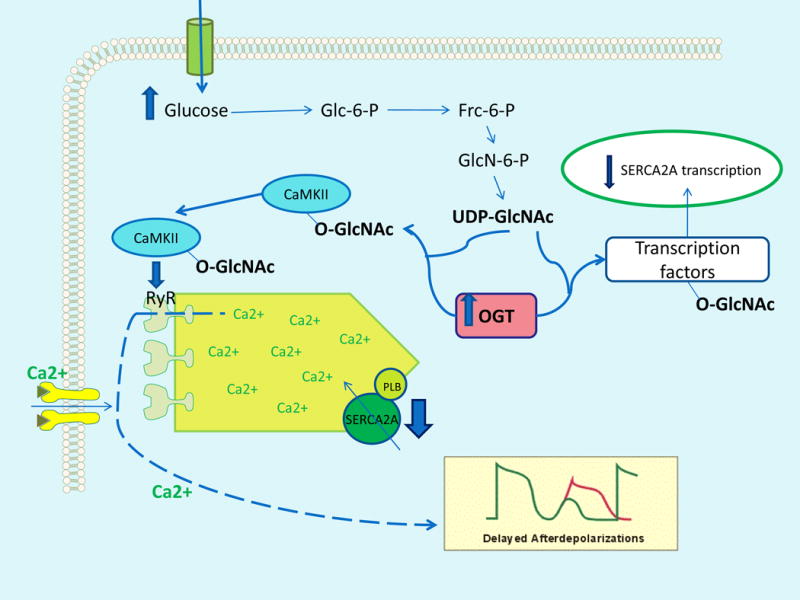
Increased intracellular glucose flux provides more substrate for the enzyme O-GlcNAc-transferase (OGT). This increases O-GlcNAc modification of calcium/calmodulin-dependent protein kinase IIδ (CaMKII), causing autonomous CaMKII activation. CaMKII increases intracellular Ca++ by phosphorylating ryanodine receptor 2 (RyR). OGT also modifies transcription complex factors regulating expression of sarcoplasmic reticulum Ca2+-ATPase (SERCA2), reducing SERCA2A expression and contributing toincreased intracellular Ca++. Increased O-GlcNAc modification of these proteins causes delayed afterdepolarizations in cardiomyocytes. PLB, phospholamban.
