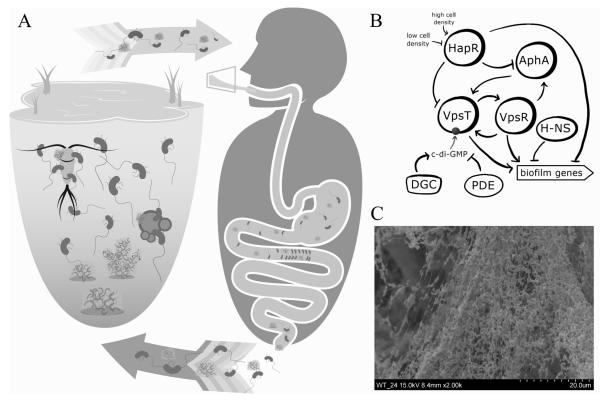
Figure 1. Role of biofilms in V. cholerae survival, transmission, and dissemination.
A) Biofilms play an important role in V. cholerae environmental protection, transmission into the human host, and dissemination to new hosts and back into environmental reservoirs. V. cholerae can be readily found growing in biofilms in the aquatic environment, often in association with zooplankton, phytoplankton, detritus, sediment, or oceanic chitin rain. This growth mode protects from a number of environmental stressors, including nutrient limitation and predation, and allows V. cholerae to survive in the aquatic environment year round. The manual removal of biofilms and plankton-associated biofilms from the environment has been shown decrease transmission during seasonal outbreaks. Additionally, the ingestion of V. cholerae grown in biofilms allows for the delivery of both higher numbers of bacteria and hyperinfectious cells. Though the role of biofilms during host infection is still being studied, biofilm-like aggregates have been observed in patient stool and also exhibit a hyperinfectious phenotype, suggesting that biofilms not only play a role in transmission from the environment to the host, but also in the spread of cholera from host to host.
B) VpsR and VpsT are the master positive regulators of biofilm genes and positively regulate one another’s expression and genes involved in biofilm formation. VpsR additionally activates the expression of a master virulence regulator, AphA, which in turn activates VpsT expression. VpsT activity is dependent on its interaction with the small signaling molecule, c-di-GMP, which is synthesized by diguanylate cyclases (DGCs) and degraded by phosphodiesterases (PDEs). The quorum sensing regulator, HapR, represses expression of VpsR, VpsT, and AphA in response to high cell density. At low cell density, HapR is inactivated and biofilm formation is upregulated. H-NS (histone-like nucleoid structuring protein) is additional negative regulator of biofilm formation. Its repressive function is silenced by VpsT.
C) An electron scanning microscopy image of a V. cholerae biofilm shows cells encased in biofilm matrix.