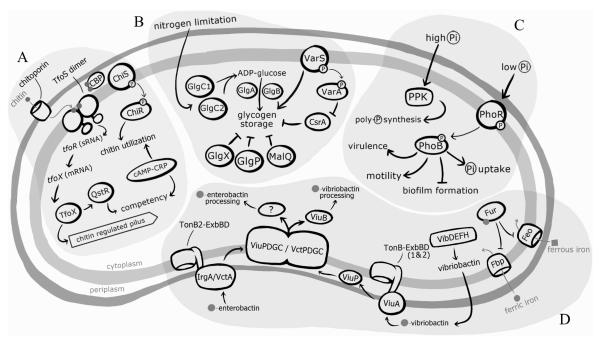
Figure 2. V. cholerae regulation of nutrient acquisition.
V. cholerae utilizes various uptake systems to acquire nutrients from the external environment. A) During chitin utilization, chitin oligomers enter the periplasm through chitoporins in the outer membrane. Once in the periplasm, chitin oligomers bind to the chitin binding protein (CBP), allowing it to release from and relive repression of the histidine kinase (HK), ChiS, which is part of a two component system (TCS). Once active, ChiS activates an as of yet unidentified response regulator (RR), ChiR, which upregulates genes involved in chitin catabolism and utilization. While ChiS is thought to also play a role in TfoS activation, the mechanism has not been identified. However, it is known that TfoS binds to chitin oligomers in the periplasm and dimerizes to become active. Once in its active conformation, TfoS upregulates the expression of the small RNA (sRNA), tfor, which, in turn, activates translation of tfox mRNA. TfoX goes on to upregulate genes involved in competency, including the chitin regulated pilus and QstR. The activation of both the chitin catabolism and competency pathways are also dependent on the cAMP-CRP complex and in the absence of this complex are repressed.
B) Glycogen storage is activated in response to nitrogen limitation. The first reaction in glycogen synthesis is catalyzed by the ADP-glucose pyrophosphorylase enzymes GlgC1 and GlgC2, which generate ADP-glucose from ATP and glucose-1-phosphate. Subsequently, the enzymes GlgA and GlgB build glycogen by forming α-1,4 and α-1,6 linkages, respectively, between ADP-glucose monomers. Glycogen breakdown is initiated by three enzymes: the glycogen debranching GlgX, the maltodextrin phosphorylase GlgP, and the 4-α-glucanotransferase MalQ. Additionally, in response to unknown environmental stimuli the TCS VarSA is activated and has been shown to enhance glycogen storage and post-transcriptionally repress the global transcriptional regulator, CsrA.
C) Environmental inorganic phosphate (Pi) levels regulate a number of cell processes in V. cholerae. When Pi is high, V. cholerae initiates the biosynthesis of large amounts of inorganic polyphosphate (poly-P), composed of long chains of linked Pi, via the polyphosphate kinase, PPK. When Pi is limited the TCS PhoBR is activated and regulates a number of cellular processes, including virulence, motility, biofilm formation, and Pi uptake.
D) V. cholerae uses a number of mechanisms to facilitate iron acquisition. Iron uptake is regulated by the iron-dependent regulator, Fur. When iron levels are high, Fur complexes with ferrous iron (Fur-Fe2+) and directly binds to conserved regions on the genome, called Fur boxes, to regulate the transcription of target genes. The Fur-Fe2+ complex upregulates genes involved in iron storage, metabolism, and antioxidant defense and represses iron uptake genes, including the genes encoding the Feo and Fbp transport systems, which facilitate uptake of ferrous and ferric iron, respectively. Under iron limited conditions, V. cholerae produces and secretes the siderophore vibriobactin via the VibBDEFH system. Ferric vibriobactin is imported back into the cell via the outer membrane protein ViuA and both of V. cholerae’s TonB-ExbBD complexes. Ferric vibriobactin is then transported through the periplasm to the inner membrane by the periplasmic binding protein, ViuP, and then across the inner membrane by two transport systems, ViuPDGC and VctPDGC. The cytoplasmic esterase, ViuB, processes ferric vibriobactin and removes the iron from the siderophore so that it may used within the cell. V. cholerae can import siderophores produced by other bacteria, including eneterobactin, which is recognized by two enterobactin receptors, IrgA and VctA, and then transported across the outer membrane with energy supplied by the TonB2-ExbBD complex, followed by shuttling across the inner membrane by the transport systems ViuPDGC and VctPDGC. The enzyme responsible for processing ferric enterobactin in V. cholerae has not been identified.