Figure 4.
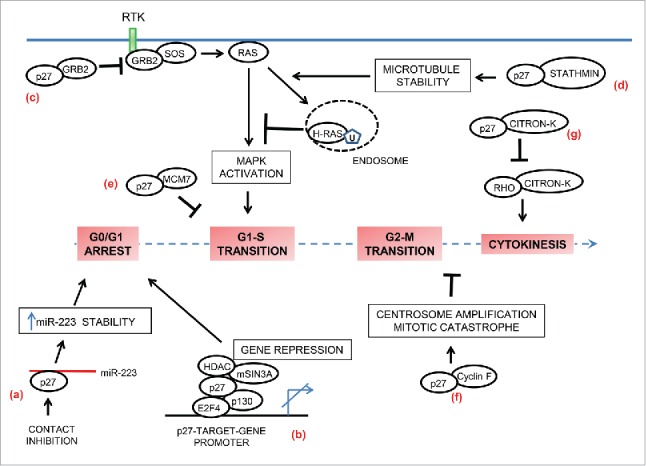
p27 in cell-cycle regulation. In addition to inhibition of cyclin-CDK activities, p27 also contributes to regulation of the cell-cycle by non-canonical means. (a) p27 is a RNA-binding protein. In contact-inhibited cells, cytoplasmic p27 directly binds and stabilizes miR-223. High levels of miR-223 then target E2F1 mRNA and thereby promote cell cycle arrest. (b) p27 also contributes to G0-G1 arrest by functioning as a transcriptional co-repressor in a CDK-independent fashion. On the promotors of specific target-genes it associates with p130/E2F4 complex and facilitates gene repression by recruiting co-repressors such as HDAC1 and mSIN3A. (c and d) p27 controls activation of MAPK pathway in a CDK-independent manner to regulate cell cycle entry. (c) In mitogen-stimulated quiescent cells, p27 is rapidly exported to the cytoplasm where it binds free GRB2 to limit GRB2-SOS complex formation and thus has the potential to attenuate MAPK activation. (d) In mitogen-stimulated cells, p27 sequesters stathmin to counteract its microtubule-destabilizing activity. The resulting enhanced microtubule-stability facilitates increased endocytic-trafficking of H-Ras and its ubiquitination, leading to attenuation of H-Ras –MAPK signaling and inhibition of cell cycle entry. (e) p27 interacts in a CDK-independent fashion with MCM7, a key component of the pre-replication complex that is assembled at the origins of replication. This interaction might prevent premature firing of the origins in late G1. (f) p27 sequesters cyclin F in a CDK-independent manner and antagonizes the cyclin F-mediated degradation of CP110 resulting in centrosome amplification and mitotic catastrophe. (g) Citron kinase (Citron-K) is essential for cytokinesis; p27 interferes with normal cytokinesis by interacting with citron-K and preventing its activation by RhoA.
