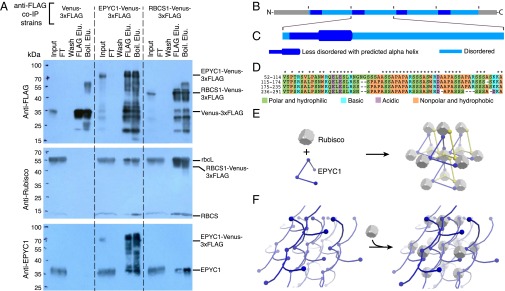
Fig. 4.
EPYC1 forms a complex with Rubisco. (A) Anti-FLAG coimmunoprecipitation (co-IP) of WT cells expressing Venus-3×FLAG, EPYC1-Venus-3×FLAG, and RBCS1-Venus-3×FLAG. For each co-IP, the input, flow-through (FT), fourth wash (wash), 3×FLAG elution (FLAG Elu.), and boiling elution (Boil. Elu.) were probed with anti-FLAG, anti-Rubisco, or anti-EPYC1. Labels on the right show the expected sizes of proteins. (B) Analysis of the EPYC1 protein sequence showing that EPYC1 consists of four nearly identical repeats. (C) Each repeat has a highly disordered domain (light blue) and a less-disordered domain (dark blue) containing a predicted alpha-helix (thicker line) rich in charged residues. (D) Amino acid alignments of the four repeats. Asterisks indicate residues that are identical in all four repeats. (E and F) Two models illustrate how EPYC1 could bind the Rubisco holoenzyme in a manner compatible with the observed packing of Rubisco in the pyrenoid. (E) EPYC1 and Rubisco could form a codependent network. If each EPYC1 can bind four Rubisco holoenzymes, and each Rubisco holoenzyme can bind eight EPYC1s, eight EPYC1 proteins could connect each Rubisco to twelve neighboring Rubiscos. (F) EPYC1 could form a scaffold onto which Rubisco binds. Both arrangements could expand indefinitely in every direction. For clarity, the spacing between Rubisco holoenzymes was increased and EPYC1 is depicted in both yellow and blue.