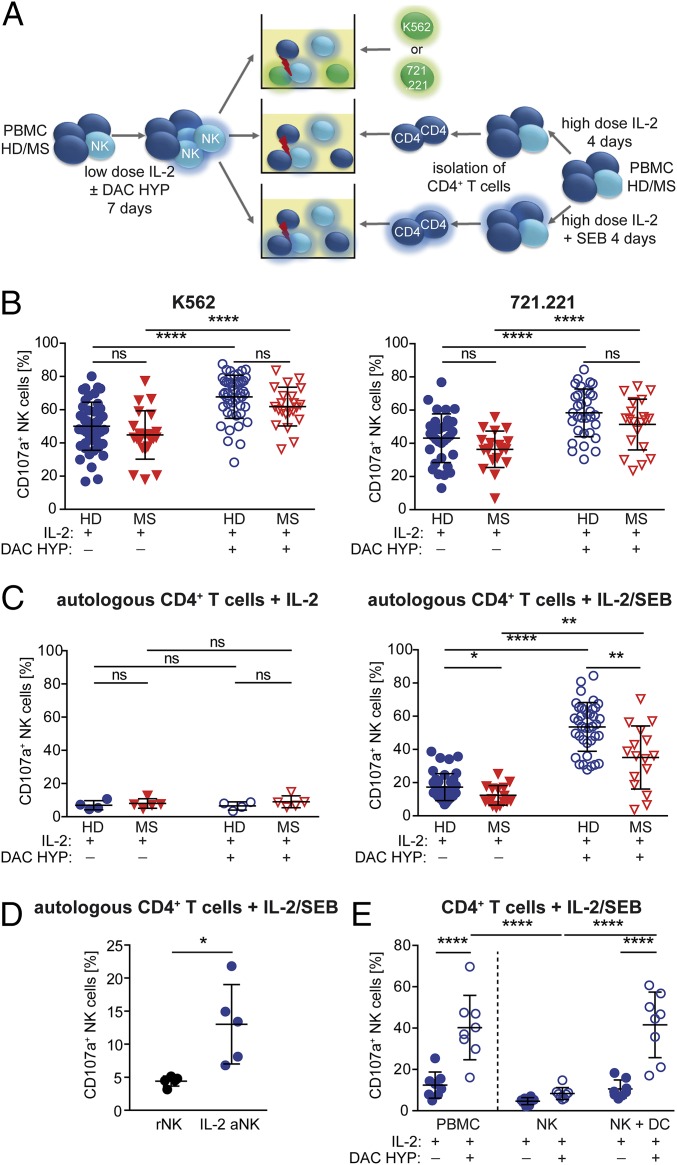
Fig. 4.
Impact of IL-2R modulation on NK-cell cytolytic activity. (A) Experimental setup: CD4+ T cells were isolated from PBMCs of HDs or therapy-naïve stable MS patients stimulated for 4 d with IL-2 with or without SEB. For NK-cell activation, PBMCs of HDs (blue circles) or MS patients (red triangles down) were stimulated for 7 d with IL-2 with or without DAC HYP. Release of cytolytic granules (degranulation) of IL-2–stimulated (closed symbols) or IL-2/DAC HYP-stimulated (open symbols) CD3−CD56+ NK cells in response to K562 (HD, n = 24; MS, n = 22) (B, Left), 721.221 (HD, n = 32; MS, n = 20) (B, Right), and autologous IL-2–stimulated (HD, n = 4; MS, n = 5) (C, Left) or IL-2/SEB-stimulated (HD, n = 38; MS, n = 16) (C, Right) CD4+ target cells was analyzed. (D) Degranulation of resting (black circles) and IL-2–activated (blue circles) NK cells (n = 5 HDs) in response to autologous IL-2/SEB-stimulated CD4+ T cells. (E) PBMCs, NK cells, and NK cells plus DCs (n = 8 HDs) were stimulated for 7 d with IL-2 with or without DAC HYP, and degranulation in response to IL-2/SEB-activated CD4+ target cells was determined. D’Agostino–Pearson omnibus normality test was performed to test for Gaussian distribution. Depending on the result, unpaired Student’s t test or Mann–Whitney test was used to compare means between two independent groups, whereas paired Student’s t test or Wilcoxon matched-pairs signed rank test was used for different treatments within the same patient group. *P < 0.05; **P < 0.01; ****P < 0.0001.