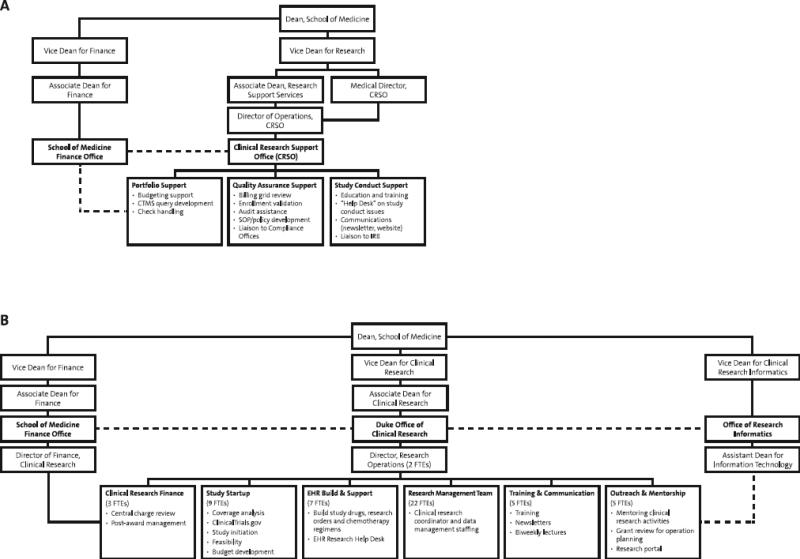
Figure 1. Organization of the Initial Clinical Research Support Office (A) and the Subsequent Unified/Expanded Duke Office of Clinical Research (B).
The Duke Office of Clinical Research replaced the Clinical Research Support Office in 2012, offering added services and greater harmonization of administrative support. The Duke Office of Clinical Research liaises with multiple other central offices, as well as faculty, staff, and students across Duke Medicine and Duke University. Investigators and research teams are supported throughout study planning, conduct, and close-out. CRSO, Clinical Research Support Office; CTMS, clinical trial management system; EHR, electronic health record; FTE, full-time equivalent; IRB, institutional review board; SOP, standard operating procedure.