Fig. 1.
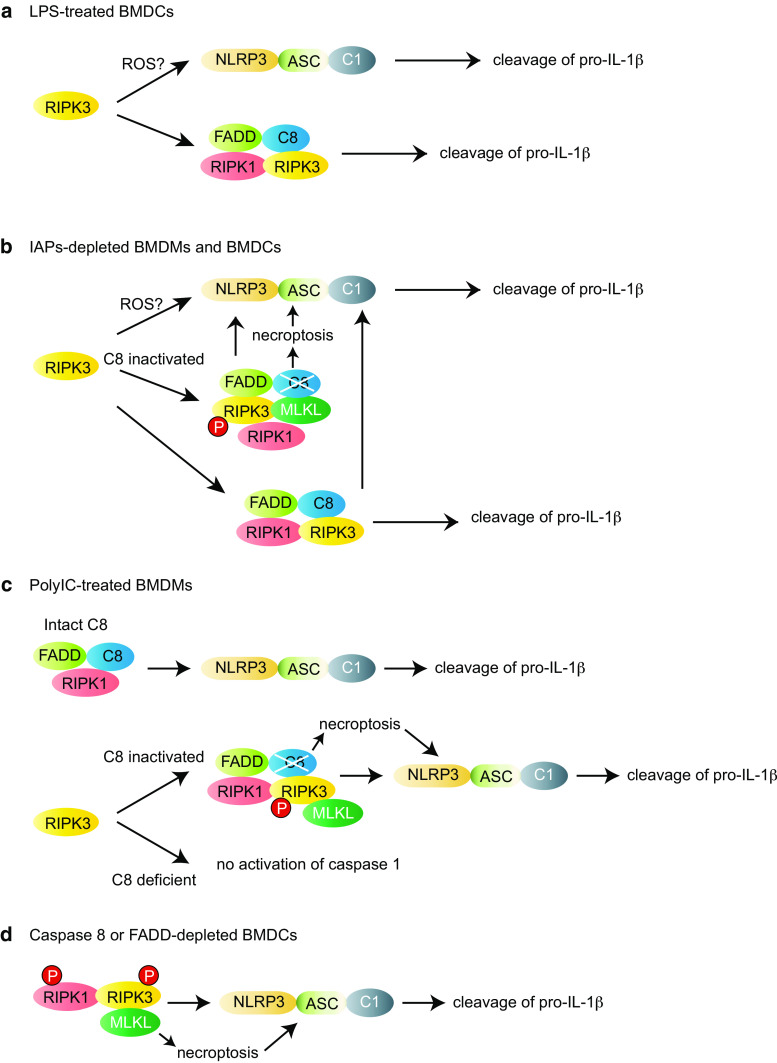
The different modes of RIPK3-mediated NLRP3 inflammasome activation. a In BMDCs, RIPK3 promotes activation of caspase 1 (C1) in response to LPS alone, possibly through ROS production. In addition to caspase 1, RIPK3 promotes caspase 8 activation through the ripoptosome, which directly cleaves pro-IL-1β. b Depletion of IAP proteins induces IL-1β secretion through robust activation of caspase 1 and caspase 8 in LPS-primed BMDMs and BMDCs. In contrast to necroptosis, the kinase activities of RIPK1 and RIPK3 are dispensable for pro-IL-1β processing through caspase 1 and caspase 8. However, when caspase 8 (C8) activity is blocked, the RIPK3 kinase activity and MLKL becomes essential to stimulate the NLRP3 inflammasome activation. c In BMDMs, poly(I:C) treatment stimulates TLR3 and TRIF, leading to FADD, RIPK1 and caspase 8 (C8)-dependent NLRP3 inflammasome activation. RIPK3 is not required for this response. However, when caspase 8 activity is blocked, RIPK3 kinase activity and MLKL phosphorylation become essential for NLRP3 inflammasome activation. In addition, RIPK3-dependent NLRP3 inflammasome activation requires caspase 8 scaffold function, since TLR3 can no longer stimulate NLRP3 inflammasome when caspase 8 is missing. d LPS-primed caspase 8 or FADD-deficient BMDCs produce increased levels of IL-1β through enhanced caspase 1 activation. This response requires RIPK1 and RIPK3 kinase activities and MLKL. MLKL activation through the RIPK3 kinase activity may directly activate the NLRP3 inflammasome. Alternatively, MLKL might enhance necroptosis, leading to DAMPs release or K+ efflux [102] and subsequent NLRP3 inflammasome activation. P in a circle indicates that the kinase activity of RIPK1 or RIPK3 is required