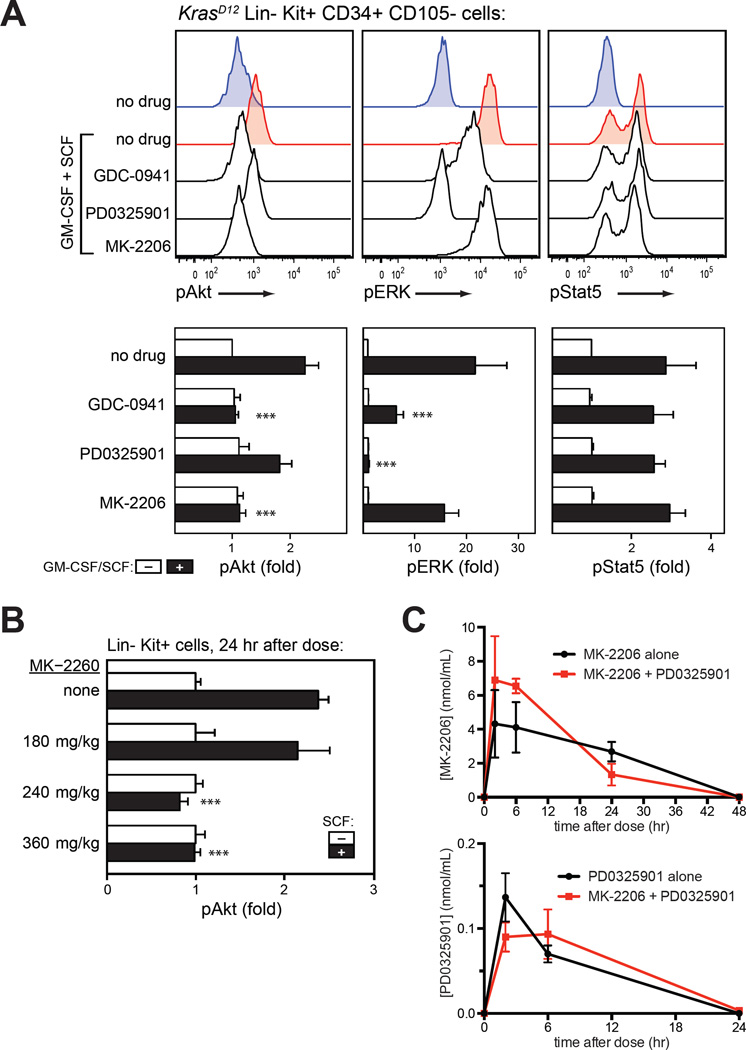
Figure 3. Pharmacologic properties of MK-2206.
(A) In vitro activity. Phosphorylation of Akt (S473), ERK (T202/Y204) and STAT5 (Y694) measured by intracellular flow cytometry in primary KrasD12 bone marrow cells. After harvest, cells were incubated with GDC-0941 (10 µM), the MEK inhibitor PD0325901 (10 µM; PD901), the Akt inhibitor MK-2206 (10 µM), or DMSO (0.001%) for 20 minutes prior to a 10-minute stimulation with GM-CSF (10 ng/mL) and stem cell factor (100 ng/mL). Lin− Kit+ CD34+ CD105− myeloid progenitor cells are shown. Data from replicate experiments appear in the lower panels. Basal signal levels (white) and stimulated levels (black) are shown for each drug treatment. Fold change in median fluoresence intensity (MFI) is displayed. Data from 5 indepdendent replicates on different days were analyzed using a linear model for MFI as predicted by stimulus, drug, replicate, and the drug-stimulus interaction, with log-transformation of MFI to correct heteroscedasticity. Asterisks indicate conditions in which the drug had a significant effect on the stimulated MFI, as indicated by the drug-stimulus interaction term (***, p<0.001); this was not statistically significant (p>0.05) where asterisks are not shown.
(B) In vivo activity. Bone marrow was harvested from mice 24 h after MK-2206 administration, immediately stimulated with SCF, fixed, and analyzed for Akt phosphorylation (S473) by intracellular flow cytometry. Data are displayed and analyzed as in panel A.
(C) Pharmacokinetic profile of MK-2206 and PD0325901 in plasma on the eighth day of treatment with either agent alone or the combination. Drug exposure was not affected by combination therapy.