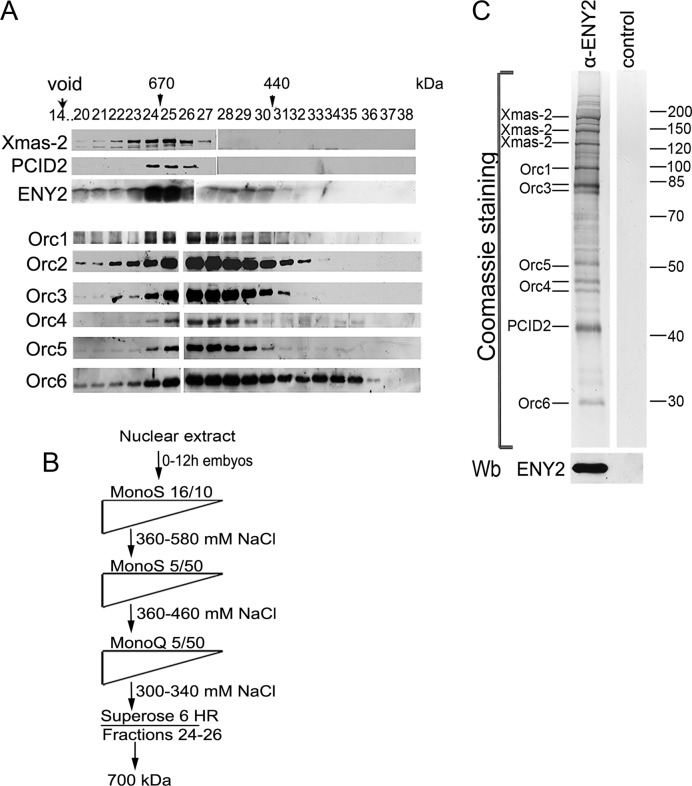
Figure 1.
Purification of TREX-2. (A) Crude embryonic extract was fractionated on a Superose 6 gel filtration column, and the fractions were analyzed for the presence of TREX-2 and ORC components by western blotting with corresponding antibodies (indicated on the left). Fraction numbers and positions of molecular weight markers (arrowheads) are indicated on the top. Void volume was eluted in fraction 14. (B) Schematic representation of TREX-2 purification procedure. Drosophila embryonic nuclear extract was fractionated by conventional chromatographic methods, including gel filtration on a Superose 6 HR column at the last step. Fractions with a molecular weight of about 700 kDa were collected and incubated with affinity purified anti-ENY2 rabbit polyclonal antibodies covalently coupled to protein A-Sepharose beads. The precipitate was washed, eluted with acidic glycine, resolved by SDS-PAGE and its components were identified by MALDI-TOF MS. (C) Coomassie staining of the purified TREX-2. Proteins eluted from the immunosorbent were resolved by 10% SDS-PAGE and Coomassie stained to be analyzed by mass spectrometry. The ENY2 subunit was detected by western blotting (Wb). The control immunoprecipitate of the same material with preimmune IgG is shown on the right.