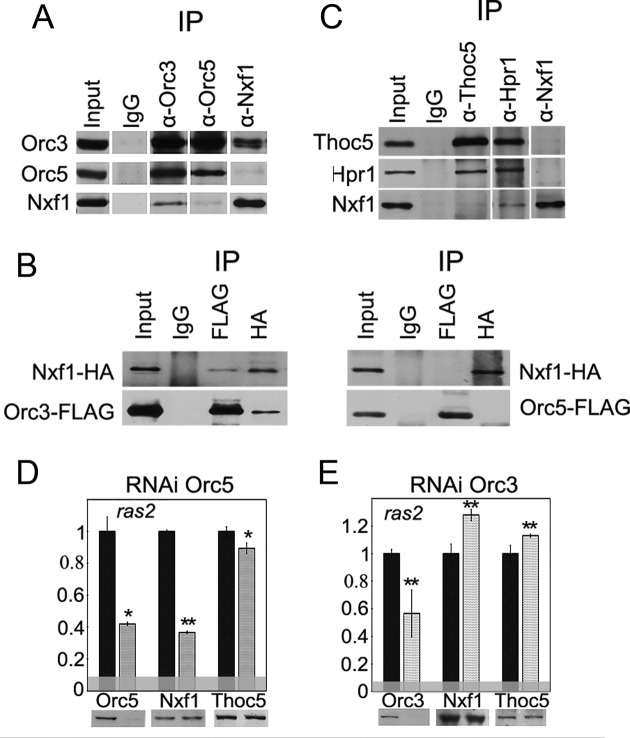
Figure 4.
ORC interacts with Nxf1 export receptor. (A) Co-immunoprecipitation of Nxf1 with Orc3 and Orc5 subunits from the nuclear extract of Drosophila embryos. (B) The recombinant Nxf1 and Orc3 proteins interact with each other. HA-tagged Nxf1 was co-expressed in S2 cells together with the FLAG-tagged ORC subunits and their interaction was verified in the co-immunoprecipitation experiments with the antibodies against FLAG or HA epitopes. (C) Co-immunoprecipitation of Nxf1 with TREX subunits Thoc5 and Hpr1 from the nuclear extract of Drosophila embryos. In (A and C), immunoprecipitation was performed with the affinity purified polyclonal antibodies against the corresponding proteins (indicated at the top) or the normal rabbit IgG coupled to the protein A-Sepharose beads. Equivalent amounts of the input fraction and of the proteins bound to the immunosorbent (IP) were loaded. (D and E) The effect of the RNAi knockdown of (D) Orc5 or (E) Orc3 on the association of Nxf1 and Thoc5 with ras2 mRNA assayed by the RIP. Antibodies used for the immunoprecipitations and the levels of the corresponding proteins in the extract are shown below the panels. Bars indicate the levels of ras2 mRNA precipitated by antibodies from Orc5 or Orc3 knockdown extracts (gray bars) normalized to the amount of the precipitate from the GFP knockdown extracts (black bars). The horizontal gray band indicates the level of ras2 mRNA co-precipitated with IgG.