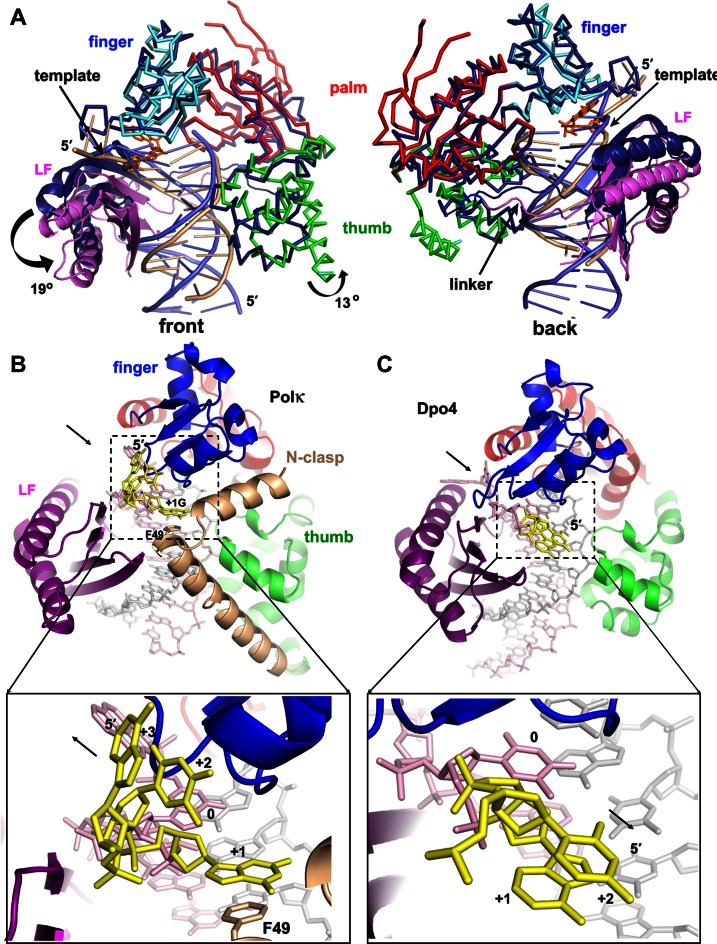
Figure 5.
Domain/DNA movements and the role of N-clasp: comparison of polκ with Dpo4. (A) Superposition of polκ and Dpo4 (1JX4). The proteins are mainly in Cα traces. Dpo4 is in dark blue, with DNA in light blue, and polκ is in the same domain colors as in Figure 2A, with DNA in beige. LF domains are highlighted in ribbons. The N-clasp domain was removed for clarity. The rotation direction and angles are in the front view (left panel). Arrows in the back view (right panel) indicate the shifting site of the template strand near the active site and the movement of the linker of polκ relative to Dpo4. (B) N-clasp is holding the finger/LF/thumb domains of polκ in position as a scaffold and adding additional contacts to the single-stranded template (yellow) to form a 3-way stabilization. The arrow in the upper panel indicates the dissociation of LF from the core; the lower panel's arrow indicates that the ss template strand extends from the active site for continuous replication. (C) Dpo4 structure (1JX4) in the same view. The N-clasp domain support for the ss template strand is absent in Dpo4. The black arrow in the upper panel indicates the association of LF with the core; the lower panel's arrow indicates the ss template strand turns back toward the major groove of the DNA helix.