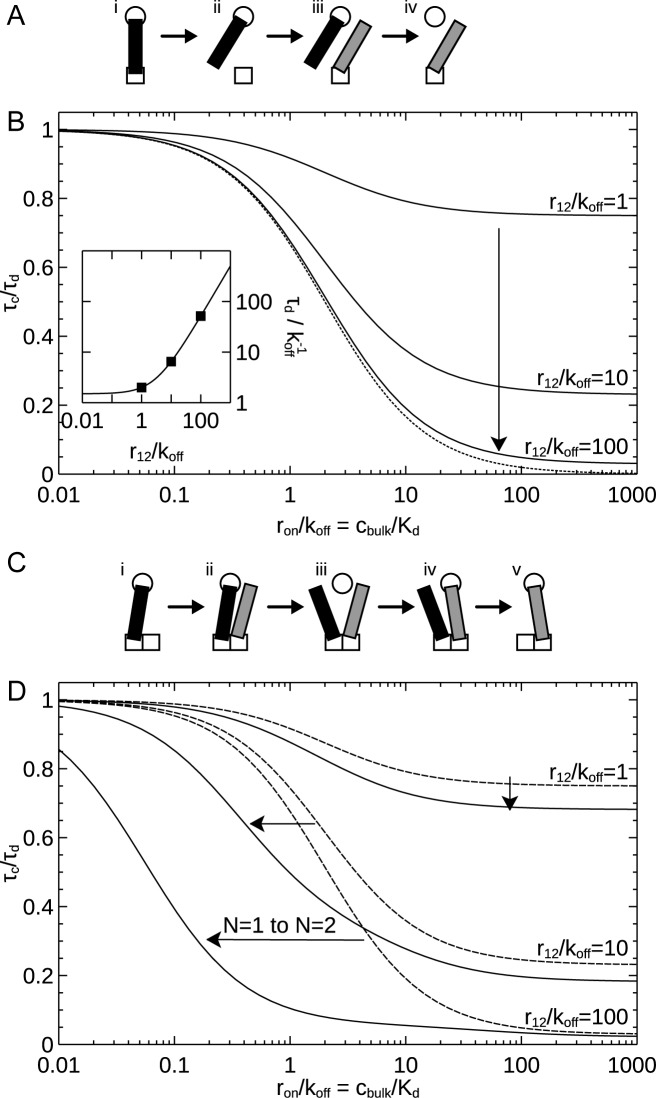
Figure 4.
Effect of multiple binding sites on stability under competition. The exchange time (τc) is normalized to the dissociation time under dilution (τd), and is shown as a function of the binding rate of competitors from solution relative to the unbinding rate (ron/koff) or, equivalently, the bulk concentration of competitors over the dissociation constant (cbulk/Kd). (A) Schematic illustrating the exchange mechanism available in the case of a single primary binding site. (gray) competing molecule. Naturally, the same process can take place on the secondary binding site. (B) Exchange time with one primary binding site (N = 1). Arrow shows direction of increased stability under dilution (r12/koff). Results calculated using Equations (4) and (6). (Dotted line) Approximation valid for a complex very stable against dilution (large r12/koff), calculated from Equation (7). (Inset) Dissociation time under dilution duplicated from Figure 3B. Symbols indicate parameters (values of r12/koff) considered in the main graph. (C) Schematic illustrating the novel mechanism present with several primary binding sites (N > 1). (D) Exchange time with two primary binding sites (N = 2). (Solid lines) Two primary binding sites. (Dashed lines) Results for one primary binding site duplicated from panel B; arrows indicate change from one to two sites. Results for N = 2 calculated using SD Equation (S13–S15).