Figure 6.
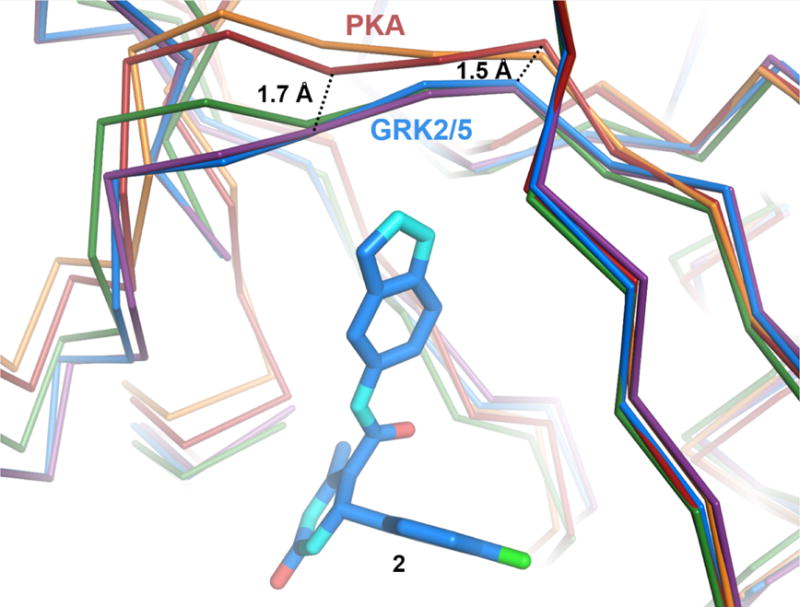
Structural differences in the hinge regions of PKA, GRK2, and GRK5. Cα traces of PKA bound to AMPPNP (PDB entry 4HPT, red) or balanol (PDB entry 1BX6, orange), superimposed onto GRK2·compound 2 (PDB entry 4PNK, blue). GRK2·12n (purple) and GRK5·12h (green) are also shown for comparison. Hinge residues that form hydrogen bonds with the indazole nitrogens of compound 2 and its derivatives are 1.5–1.7 Å closer to the inhibitor in the structures of GRK2 and GRK5 relative to those of PKA.
