Figure 2. Two distinct cases of epitope targeting.
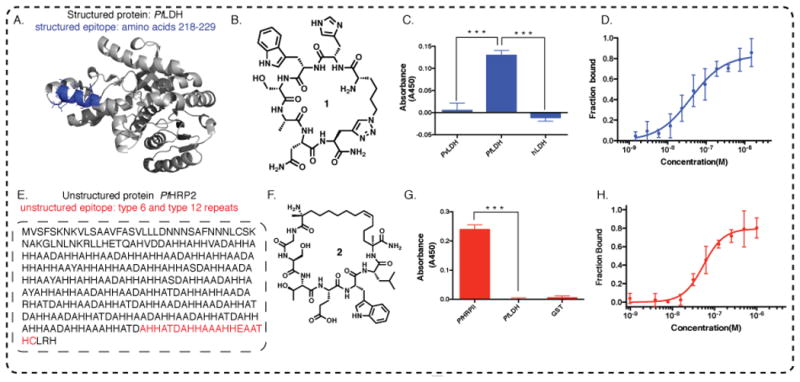
A. A region from the structured PfLDH protein that uniquely distinguishes PfLDH relative to other LDH variants, is highlighted in blue. B. The molecular structure of 1, the consensus PCC developed to bind to the blue highlighted epitope of PfLDH; C. Peptide 1 selectively distinguishes PfLDH from PvLDH (v=vivax) and hLDH (h=human) in single point ELISA (proteins spiked into buffer solution at 100 nM concentration). D. Fluorescein tagged 1 is titrated with recombinant PfLDH and the fluorescence polarization is measured, yielding a KD of 40.63 ± 9.27 nM. E. Targeting a universally conserved epitope of intrinsically unstructured PfHRP2 protein. One recombinant (r) PfHRP2 amino acid sequence is shown. The epitope targeted is highlighted in red. F. Molecular structure of 2, the consensus PCC developed to bind to the highlighted epitope from PfHRP2. G. Selective binding of 2 to 20 nM GST-PfHRP2, showing negligible cross-reactivity with 20 nM GST-PfLDH, a related target from Plasmodium falciparum species, and with 20 nM GST protein; H. Peptide 2 binds to recombinant PfHRP2 with a KD of 54.26 ± 12.05 nM, as measured by fluorescence polarization.
