Figure 1. Disease-causing Mutations in Human TBK1.
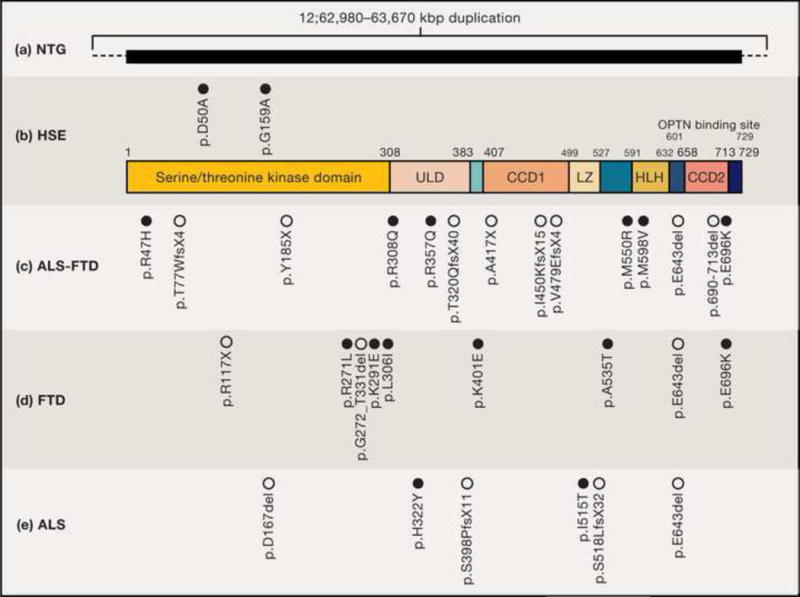
TBK1 is an 82 kDa, 729 amino-acid protein composed of a kinase domain, an ubiquitin-like domain (ULD), and coiled-coiled domains 1 and 2 (CCD1; CCD2). The kinase domain is critical for the phosphorylation of various substrates such as IRF3 [15], whereas the ULD domain regulates kinase activation and interactions with other proteins in the TBK1 pathway [112]. The CCD1 domain harbors leucine zipper (LZ) and helix-loop-helix (HLH) domains which specifically control dimerization. The C-terminus CCD2 harbors an adaptor-binding motif facilitating the interaction of TBK1 with its adaptors TANK, NAK–associated protein (NAP1), and Similar to NAP1 TBK1 Adaptor (SINTBAD) [89]. Germline human TBK1 mutations have been reported to be disease-causing in (a) normal tension glaucoma (NTG), (b) herpes simplex encephalitis (HSE), (c) amyotrophic lateral sclerosis-frontotemporal dementia ALS-FTD, (d) FTD and (e) ALS : these mutations are shown with respect to their amino acid positions within the TBK1 protein. The black horizontal box in (a) indicates duplications in kb reported to include TBK1. Open circles represent LoF variants; filled circles represent missense variants. (See Table 1).
