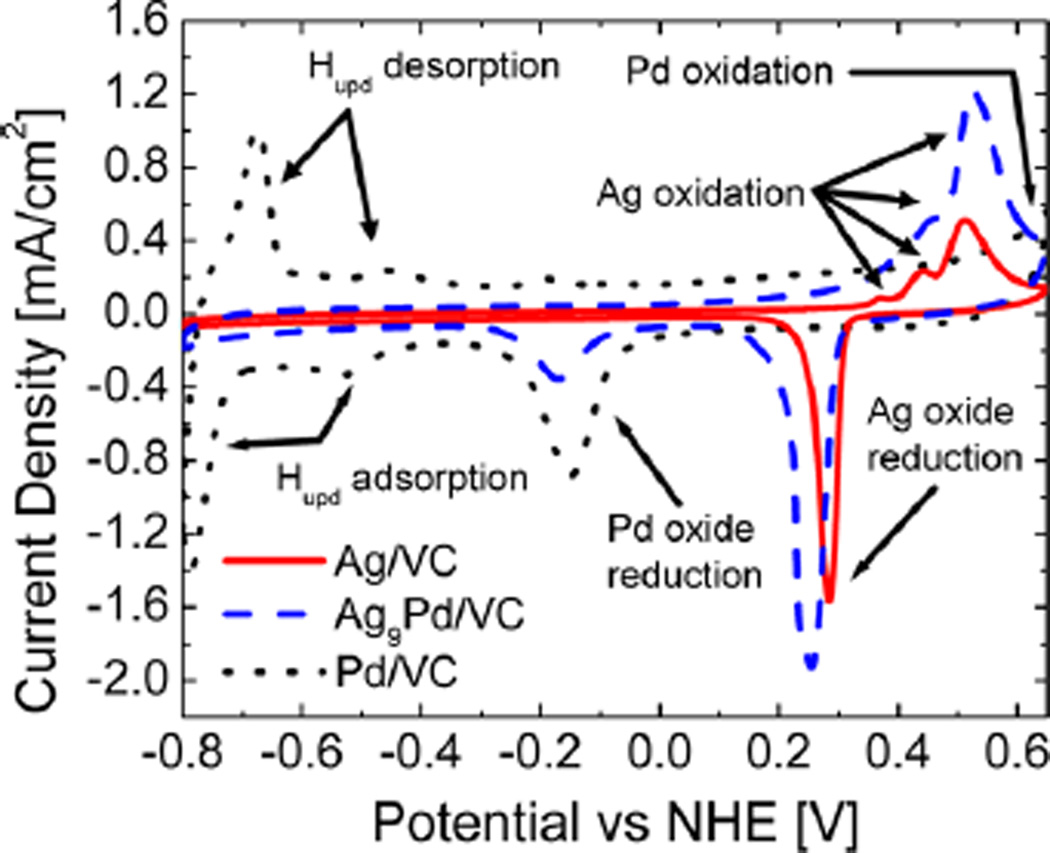
Figure 4.
Cyclic voltammograms showing the characteristic redox peaks for the alloy and pure metal catalysts supported on Vulcan XC72 carbon. The presence of both Ag and Pd oxide reduction peaks shows that the surface is composed of both metals. However, the lack of Hupd for the alloy suggests that the Pd is dispersed primarily as single atomic sites. The silver oxidation peaks from 0.3 to 0.6 V shift slightly positive for the alloy catalyst, suggesting some resistance to oxidation due to the small amount of Pd. The large reduction peak of silver oxide is shifted negative, suggesting slightly more stable oxide formation.