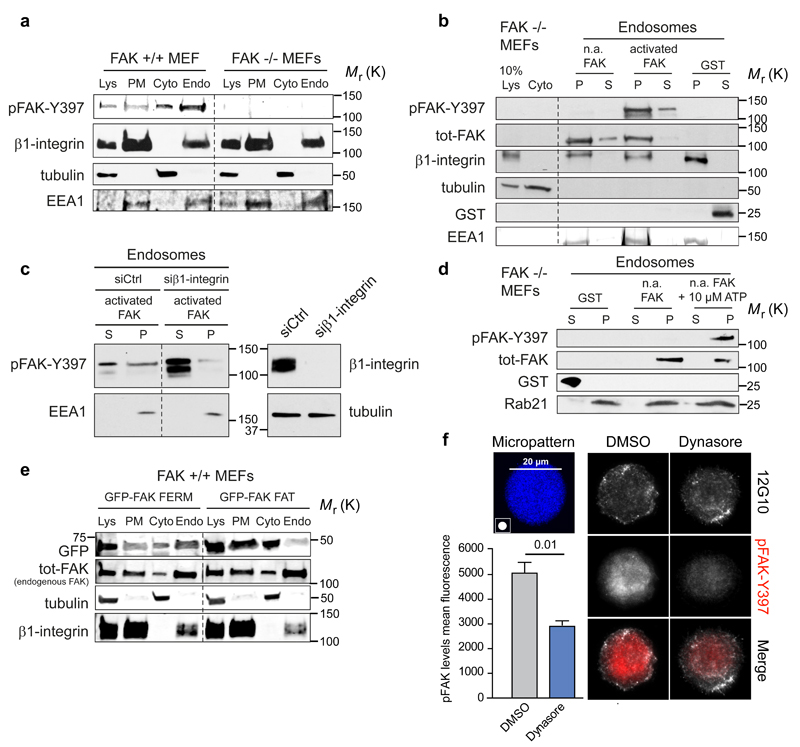
Figure 4. FAK recruitment and activation on integrin-positive endosomes.
a, Representative immunoblot of subcellular fractionation of FAK+/+ and FAK-/- MEFs (five independent experiments). b, Representative immunoblot of recombinant FAK (phosphorylated/activated or non-activated: n.a) recruitment to insoluble endosomal pellet (P) and soluble supernatant (S) fractions isolated from FAK -/- MEFs (five independent experiments). c, Representative immunoblot analysing the recruitment of recombinant FAK to purified endosomes derived from either control- or β1-integrin-silenced FAK -/- MEFs (five independent experiments). d, Representative immunoblot analysing the activation of recombinant FAK in purified endosome fractions derived from FAK -/- MEFs in the presence or absence of 10 µM ATP (five independent experiments). e, Subcellular fractionation of FAK+/+ MEFs transfected with GFP-FAK FAT or GFP-FAK FERM. Shown are representative immunoblots of GFP-FAK fragments and endogenous FAK (total-FAK) from two independent experiments. f, MDA-MB-231 cells plated on 20 μm round fibronectin-coated micropatterns (45 min) ± dynasore. Representative maximum projections and quantification of pFAK (mean fluorescence ± SEM, n=3 independent experiments, 10 cells analysed / experiment). Lys: cell lysate; PM: plasma membrane; Cyto: cytosol; Endo: Endosomal fraction. Student's two-tailed unpaired t-test P =0.01. Uncropped images of blots are shown in supplementary figure 9.