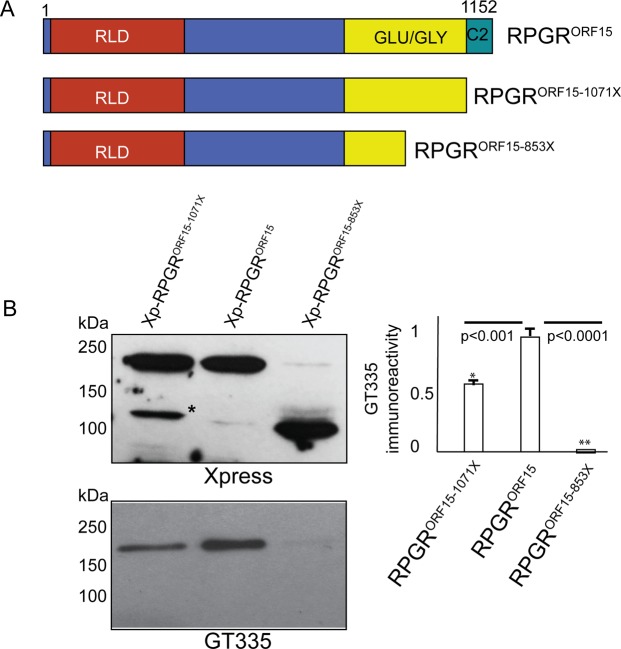
Fig. 4.
Human disease mutations in RPGRORF15 alter binding to GT335. (A) Schematic representation of the location of the human exon ORF15 mutations. RLD, RCC1-like domain; Glu-Gly, glutamic acid and glycine rich domain; C2, RPGRC2 domain. (B) hTERT-RPE1 cells were transiently transfected with constructs encoding Xpress-tagged full-length RPGRORF15 or two disease-causing mutants; RPGRORF15-1071X or RPGRORF15-853X. Equal amount of protein extracts from these cells were analyzed by SDS-PAGE and immunoblotting using anti-Xpress (upper panel) or anti-GT335 (lower panel) antibody. Asterisk indicates degraded protein product. Densitometric analysis was performed to quantify the immunoblot signal. The data are represented as ratio relative to the intensity of GT335-immunoreactivity with full-length RPGRORF15, set as 1. Error bars represent standard deviation. *P<0.001; **P<0.0001. Data are representative of three independent experiments.