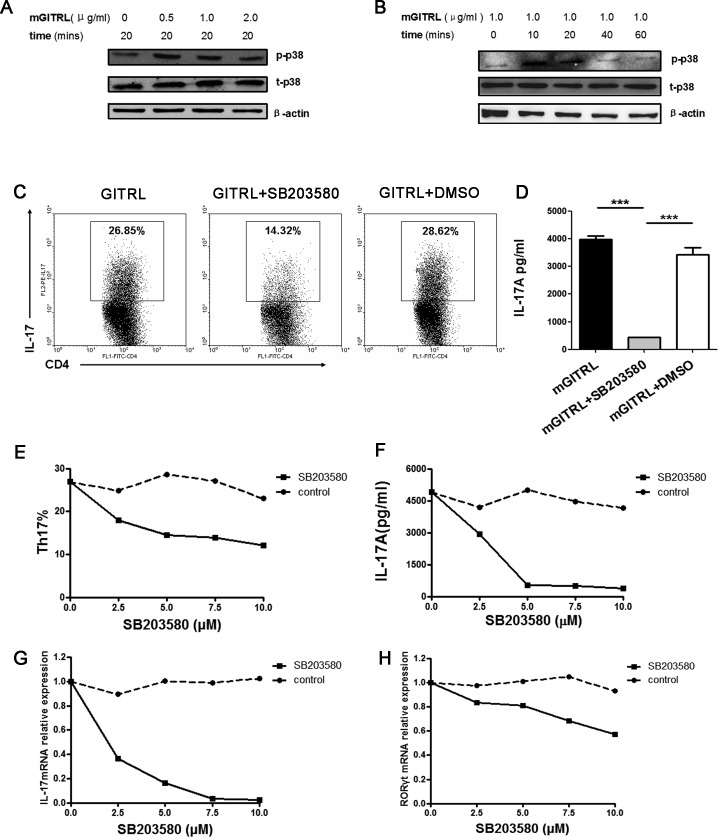
Figure 1. p38 MAPK is necessary for GITRL-induced Th17 differentiation.
A. Naïve CD4+T cells were activated by an anti-CD3 mAb (1 μg/mL) and GITRL protein (1 μg/mL) for 72 hours. The activated cells were washed and restimulated with different concentrations of GITRL protein (0, 0.5, 1.0, 2.0 μg/mL) at 37°C for 20 min. The phosphorylation of p38 MAPK was detected by Western blot. B. Naïve CD4+T cells were activated by anti-CD3 mAb (1 μg/mL) and GITRL protein (1 μg/mL) for 72 hours. The activated cells were washed and restimulated with GITRL protein (1 μg/mL) at 37°C for 10-60 min. The phosphorylation of p38 MAPK was detected by Western blot. C., D. Naïve CD4+T cells were cultured with TGF-β (2.5 ng/mL), IL-6 (30 ng/mL), IL-23 (30 ng/mL), anti-IFN-γ (5 μg/mL), anti-IL-4 (5 μg/mL) and GITRL protein (1 μg/mL) in a 24-well plate precoated with anti-CD3 mAb (1 μg/mL) for 72 hours in the presence or absence of the p38 MAPK inhibitor SB203580 (5 μM); the DMSO was the solvent of SB203580. The frequencies of Th17 cells in cultures with different treatments were analyzed by FCM C. The concentration of IL-17 in the culture supernatant was detected by ELISA D., E., F., G., H. Naïve CD4+T cells were cultured under Th17 cell differentiation conditions and treated with GITRL protein (1 μg/mL) for 72 hours in the presence of SB203580 (0, 2.5, 5, 10 μM) or the solvent control. The frequencies of Th17 cells in cultures with different treatments were analyzed by FCM E.. The concentrations of IL-17 in the culture supernatant was detected by ELISA F.; relative expression of IL-17 G. and RORγt H. mRNA were detected by qRT-PCR. Data are mean±SD. ***, P < 0.001.