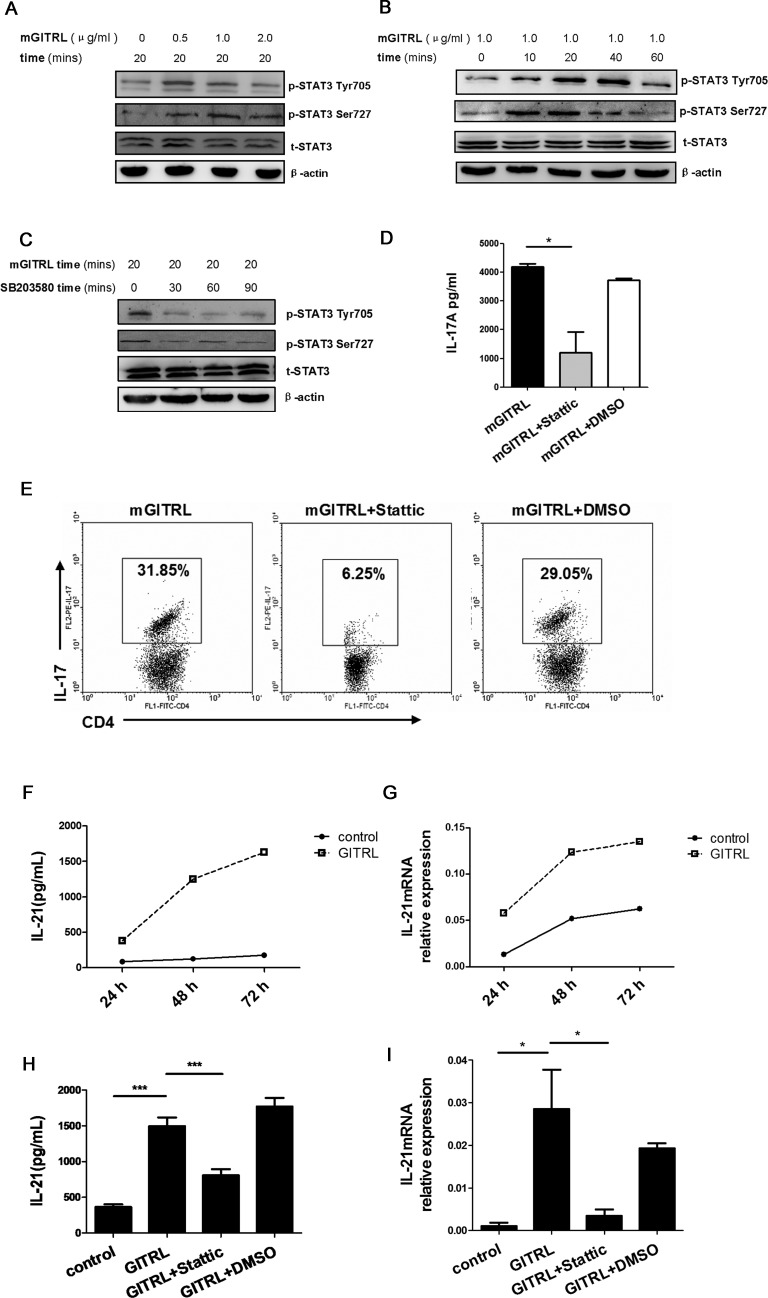
Figure 2. GITRL protein promotes the phosphorylation of STAT3 on Tyr705 and Ser727 via p38 MAPK in Th17 cell differentiation.
A. Purified Naïve CD4+T cells were activated by anti-CD3 mAb (1 μg/mL) and GITRL protein (1 μg/mL) for 72 hours. The activated cells were washed and restimulated with different concentrations of GITRL protein at 37°C for 20 min. The phosphorylation of STAT3 Tyr705 and Ser727 was detected by Western blot.B. The activated CD4+T cells were restimulated with GITRL protein (1 μg/mL) at 37°C for different lengths of time. The phosphorylation of STAT3 Tyr705 and Ser727 was detected by Western blot.C. The activated CD4+T cells were pretreated with SB203580 (5 μM) for 30, 60, 90, and 120 min and restimulated with GITRL protein (1 μg/mL) for 20 min at 37°C. The phosphorylation of STAT3 Tyr705 and Ser727 was detected by Western blot.D., E. Naïve CD4+T cells were cultured under Th17 condition as previously described and treated with GITRL protein (1 μg/mL) for 72 hours in the presence or absence of the STAT3 inhibitor Stattic (20 μM). DMSO was used as the solvent of Stattic. The concentration of IL-17 in the culture supernatant was detected by ELISA D.. The frequencies of Th17 cells in the cultures with the different treatments were analyzed by FCM E., F., G. Naïve CD4+ T cells were cultured in the condition as previously described with either GITRL protein (1 μg/mL) or control protein for 72 hours. IL-21 production F. and IL-21 mRNA expression G. was detected by ELISA and qRT-PCR, respectively, every 24 hours.H., I. Naïve CD4+ T cells were cultured in the conditions previously described and treated with either GITRL protein (1 μg/mL) or a control protein for 72 hours in the presence or absence of Stattic (20 μM). DMSO was the solvent of Stattic. IL-21 production H. and IL-21 mRNA expression I. was detected by ELISA and qRT-PCR, respectively. Data are mean±SD. *, P < 0.05; ***, P < 0.001.