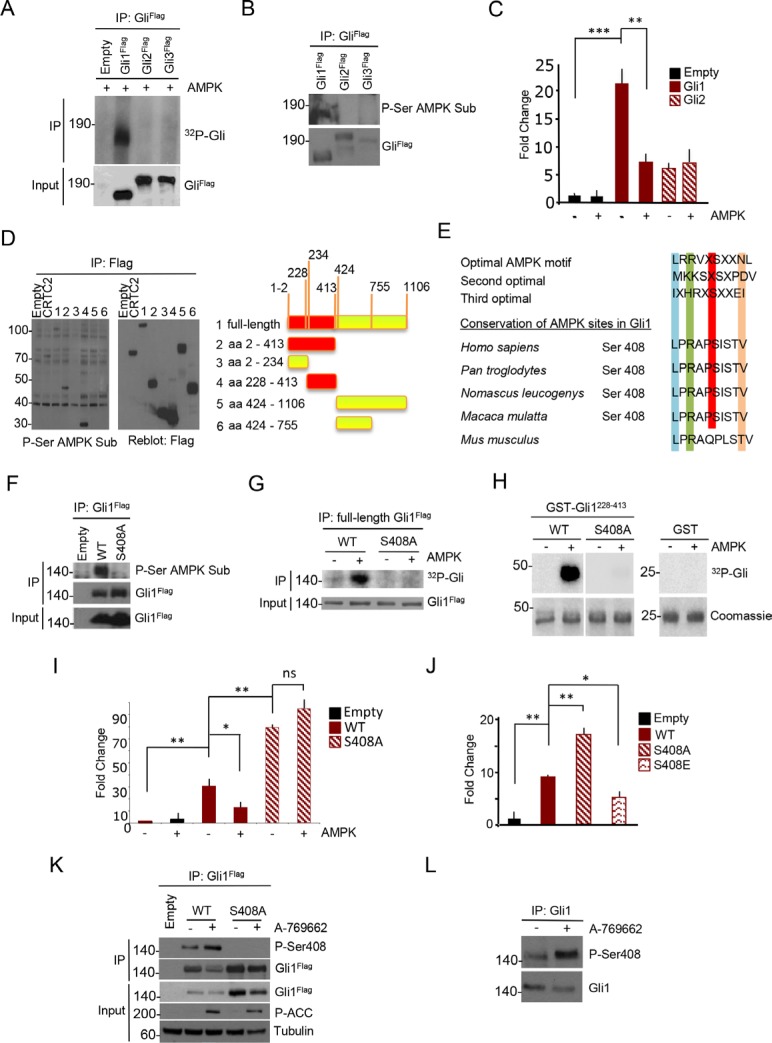
Figure 2. AMPK phosphorylates human Gli1 at Ser408.
(A) Flag-Gli1, Flag-Gli2 and Flag-Gli3 proteins were expressed in HEK293T cells, and immunoprecipitated from whole cell lysates with Flag antibody. Eluted proteins were then incubated with catalytically active AMPK protein. 32P incorporation levels were assessed by autoradiography. Gli proteins expression was evaluated by western blot analysis with Flag antibody. (B) HEK293T cells were transfected with Flag-Gli1, Flag-Gli2 and Flag-Gli3 and overexpressed proteins were purified by immunoprecipitation. Phosphorylation was assessed by immunoblotting with anti-phospho serine AMPK substrate (P-Ser AMPK Sub) antibody. Filters were reprobed with Flag antibody to detect immunoprecipitated Gli protein levels. (C) Gli-Luc reporter assay showing the effect of AMPK overexpression on Flag-Gli1 and Flag-Gli2 transcriptional activity in DAOY cells. Results are expressed as Luciferase/Renilla fold change relative to control sample. (D) Left, western blot analysis of immunoprecipitates from HEK293T cells, transfected with plasmids encoding full-length Flag-tagged Gli1 or indicated fragments. Phosphorylation of the various Gli1 regions was assessed. Flag-CRTC2 was used as positive control. Right, schematic representation of Gli1 fragments. Red: phosphorylated fragments. (E) Protein sequence alignment of primates and murine Gli1, showing a conserved AMPK phosphorylation motif around Serine 408 (Ser408). Optimal AMPK motives are shown. (F) In vivo phosphorylation assay in HEK293T cells. Flag-tagged WT or S408A mutant Gli1 proteins were overexpressed and immunoprecipitated. Phosphorylation was assessed by immunoblot with anti-phospho serine AMPK substrate (P-Ser AMPK Sub) antibody. WT and mutant Gli1 protein levels in immunoprecipitated samples and cell lysates (Input) was carried out with Flag antibody. (G) Kinase assay on WT and S408A mutant Gli1 proteins, with or without active AMPK protein. Flag-Gli1 WT and S408A mutant were expressed in HEK293T cells and immunoprecipitated. 32P incorporation was revealed by autoradiography. Gli1 proteins expression was evaluated by western blot analysis. (H) In vitro AMPK-phosphorylation assay of GST alone, recombinant GST-Gli1 228–413 WT or S408A mutant. Incorporation of 32P was determined by autoradiography and the protein levels were detected by Coomassie blue staining. (I) Gli-Luc reporter assay showing the effect of AMPK on Gli1 WT and S408A mutant activity. Results are expressed as Luciferase/Renilla fold change relative to control sample. (J) Transcriptional activity of Gli1 WT versus non-phosphorylatable and phospho-mimetic mutants. Results are expressed as Luciferase/Renilla fold change relative to control sample. (K) HEK293T cells were transfected with Flag-tagged Gli1 WT and S408A mutant and treated with A-769662 (25 μM) for 1 hour. Cell extracts were immunoprecipitated with anti-Flag antibody and Serine 408 phosphorylation was revealed with a phospho-Serine 408 (P-Ser408) antibody. Immunoprecipitated Gli1 protein levels are shown. Western blotting of cell lysates (Input) was performed with Flag, phospho-ACC (P-ACC) and tubulin antibodies. (L) Serine 408 phosphorylation of endogenous Gli1 in DAOY cells. Cells were treated with A-769662 for 1 hour and Gli1 protein was immunoprecipitated from whole cell lysates. Immunoprecipitated Gli1 protein levels are shown. Results are expressed as mean and SD of three independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 and ns (not significant) for the indicated comparisons.