Figure 4. Analysis of actionability in 171 patients with diverse cancers.
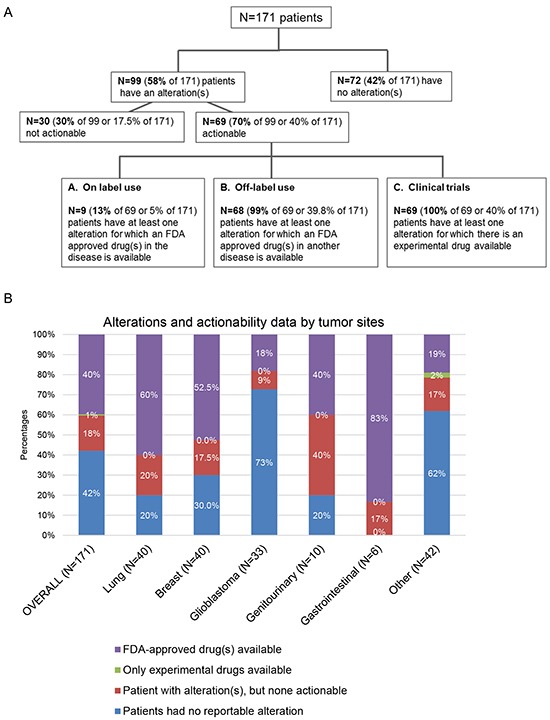
Panel A. there is some overlapping as some patients might have approved agents on and off-label, as well as experimental drugs options for their disease - patients described in box A (on label use) all also have “off label use” options and are included in box B (Off-label use). Similarly, patients described in boxes A and B all also have clinical trial options and are included in box C. All patients with actionable alterations had at least one clinical trial suggested. Panel B. displays the percentages of actionability data by tumor sites. Other included: unknown primary, n=39; melanoma, n=1; sarcoma, n=1; thymic sarcoma, n=1.
