Figure 2. Long time inhibition of one signaling activates the other pathway and restores eIF4B Ser422 phosphorylation.
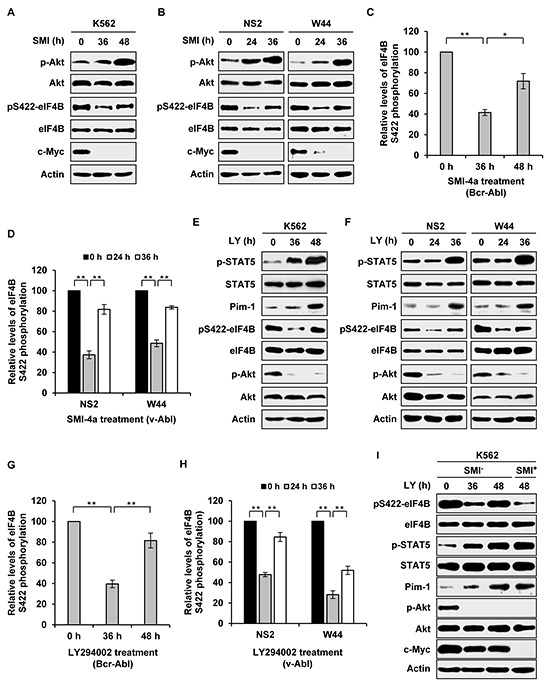
A. K562 cells were treated with 5 μM SMI-4a for indicated time. Examination of eIF4B and Akt phosphorylation was performed with indicated antibodies. B. NS2 and W44 cells were treated with 2 μM SMI-4a for indicated time. The phosphorylation of eIF4B Ser422 and Akt was analyzed as described in A. C. and D. eIF4B phosphorylation levels in A and B were quantitated by densitometry and normalized to total protein levels. The levels of eIF4B S422 phosphorylation are 100% at 0 h. Plotted are results from three independent experiments. Error bars represent SEM, n = 3 (*P < 0.05, **P < 0.01). E. and F. K562, NS2, or W44 cells were treated with 5 μM or 2 μM LY294002 for indicated time. The cell lysates were analyzed by Western blotting with indicated antibodies. G. and H. eIF4B phosphorylation levels in E and F were quantitated as described in C. I. experiments were performed as described in E. At 40 h, K562 cells treated with LY294002 were collected, washed with PBS and incubated with mixture of 5 μM LY294002 and 5 μM SMI-4a or with mixture of 5 μM LY294002 and vehicle for 8 h. Cells were analyzed for phosphorylation of eIF4B Ser422 by Western blotting.
