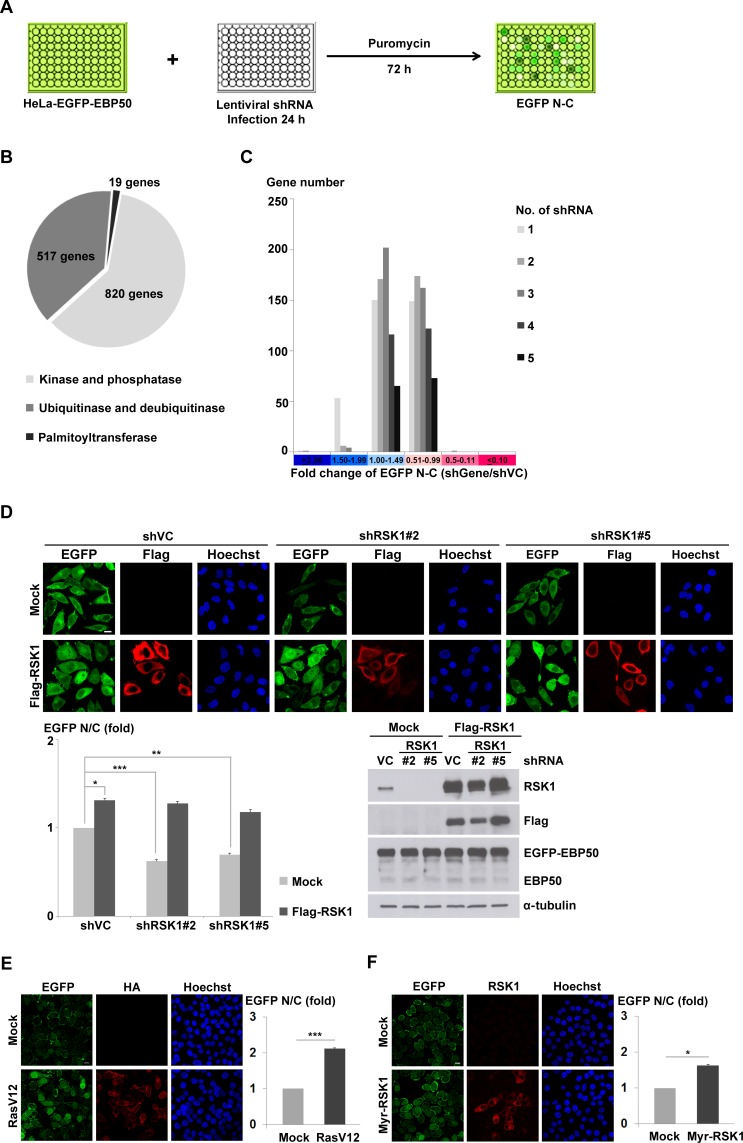
Figure 1. Ras-RSK1 signaling regulates nuclear localization of EBP50.
A. Schematic diagram of the RNAi screening that was subsequently coupled with image analysis to search for genes involved in the nucleocytoplasmic transport of EBP50. B. Totally 1356 genes composed of three major enzymatic subclasses responsible for protein phosphorylation, ubiquitination, and palmitoylation were screened for their potential effects on the nucleocytoplasmic shuttling of EBP50. C. The nuclear and cytosolic (N-C) difference of EGFP signals after normalization with that from the mock-transduced cells (shVC) was presented according to the distribution of shRNA hits in the designated categories for each gene. D. Flag-tagged mouse RSK1 (Flag-RSK1) was introduced as the RNAi-resistant rescue construct to the HeLa-EGFP-EBP50 cells which were separately infected with two independent clones of lentiviruses expressing shRNAs against RSK1. Immunofluorescence images were analyzed for N/C ratio of EGFP signal (left lower panel). Western blotting analysis confirmed the knockdown efficiency of endogenous RSK1 and successful re-expression of Flag-RSK1 (right lower panel). α-tubulin was examined as a loading control. HeLa-EGFP-EBP50 cells were transfected with a plasmid encoding constitutive active Ras (HA-RasV12) E., constitutive active RSK1 (Myr-RSK1) F., or the empty vector (mock). The cells were then fixed at 24 h post-transfection, stained with anti-HA E. or anti-RSK1 antibody F., and then images were captured and N/C ratio of EGFP signal were quantified. Scale bar: 10 μm. Data are means ± SEM of 90-200 cells. * p < 0.05, ** p < 0.01, *** p < 0.001, Student's t-test.