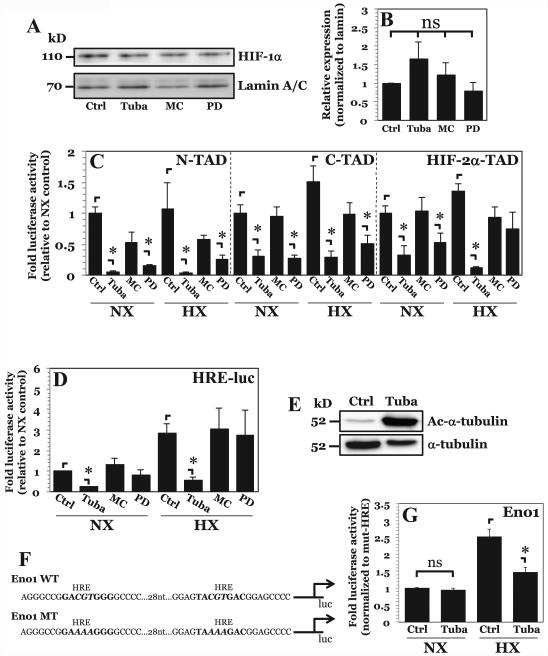
Figure 3. HDAC6 inhibition results in decreased HIF-1α activity independent of effects on protein stability.
A, B) Western blot of HIF-1α (A) and corresponding densitometric analysis of multiple independent experiments (B) after NP cell treatment with inhibitors Tubastatin A (15 μM), MC1568 (20 μM), or PD-106 (10 μM). Selective inhibition of HDAC6, class IIa HDACs, or class I HDACs had no effect on HIF-1α protein stability. C, D) HIF-1α-N-TAD, HIF-1α-C-TAD, and HIF-2α-TAD and D) HRE-luciferase reporter activity activity under normoxia and hypoxia after Tubastatin A, MC1568, or PD-106 treatment. A decrease in HIF-1α-N-TAD and HIF-1α-C-TAD activity was seen with inhibition of HDAC6 and Class I HDACs. Endogenous HIF-1α activity, however, was decreased only by HDAC6 inhibition under both normoxia and hypoxia. E) Western blot analysis of α-tubulin, a known target of HDAC6, shows significantly enhanced acetylation (Ac-α-tubulin) after treatment with Tubastatin A. F) Schematic of enolase-1 promoters used in studies. G) Enolase promoter activity, normalized to activity of promoter with mutated HRE sites, after treatment with Tubastatin A under normoxia and hypoxia. Effects of HDAC6 inhibition on promoter activity are only seen under hypoxia. Data is represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.