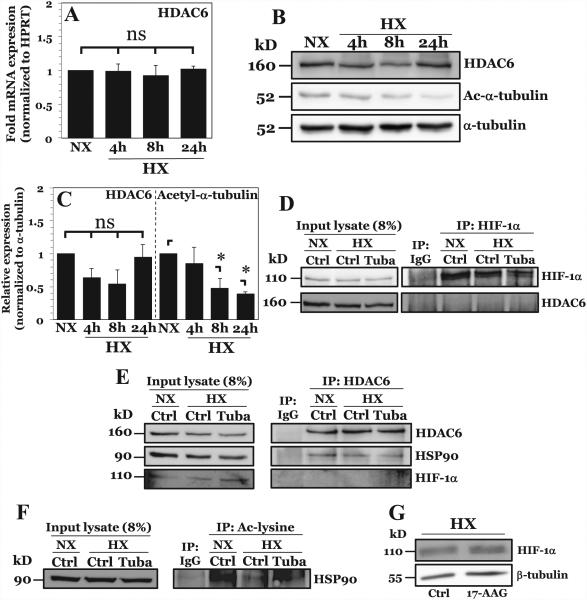
Figure 5. HDAC6 promotes HIF-1 activity in NP cells under hypoxia through positive effects on HIF-1 transcriptional cofactor HSP90.
A) mRNA expression of HDAC6 measured by qPCR after culture in HX for 4-24 hours. B, C) Western blot analysis of HDAC6 and acetylated α-tubulin (Ac-α-tubulin) (B) and corresponding densitometric quantification of experiments (C) following NP cell culture in hypoxia (HX) for 4-24 h. HDAC6 activity, but not expression, increases under hypoxia. D) Immunoprecipitation of HIF-1α in NP cells under normoxia and hypoxia. HDAC6 was unable to be co-precipitated with HIF-1α. E) Immunoprecipitation of HDAC6 in NP cells with or without Tubastatin A treatment. HSP90, but not HIF-1α, was immunoprecipitated with HDAC6 under both normoxia and hypoxia. F) Immunoprecipitation of acetyl-lysine residues in NP cells under normoxia and hypoxia with or without Tubastatin A. Acetylation of HSP90 decreases under hypoxia and increases with HDAC6 inhibition. G) Western blot analysis of HIF-1α after HSP90 inhibition by 8 hr treatment with 17-AAG (500 nM). HSP90 inhibition results in no appreciable change in HIF-1α protein levels. Quantitative data is represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.