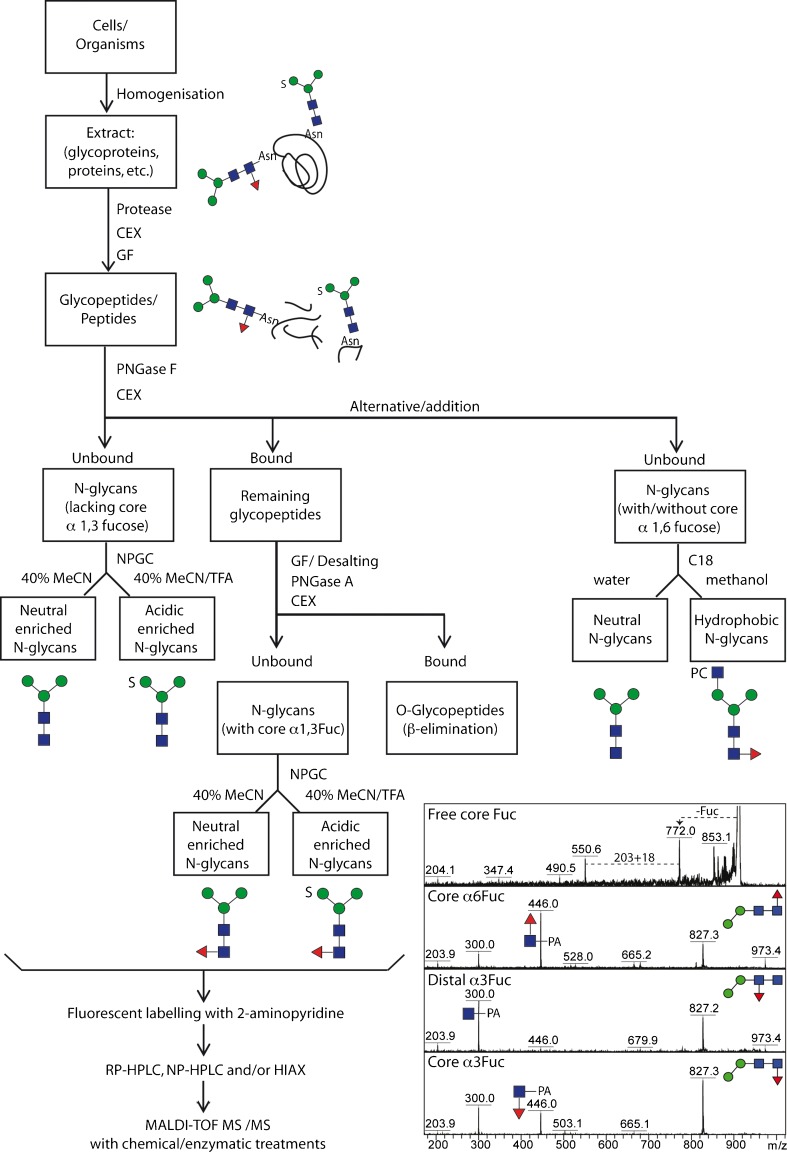
Fig. 2.
Analytical workflow for off-line LC-MALDI-TOF MS glycan analysis. The overall concept of our N-glycome analytical studies; initially, samples are proteolysed, the glycopeptides enriched by cation exchange (CEX) and gel filtration (GF) and the glycans released enzymatically, whereby PNGase A (and not PNGase F) is capable of releasing the core α1-3-fucosylated N-glycans. Subsequent sub-fractionation by non-porous graphitised carbon (NPGC) and/or reversed-phase (C18) resins result in pools differing in terms of anionic and zwitterionic modifications. Finally, all N-glycans are analysed by different types of HPLC (reversed or normal phase or hydrophilic interaction/anion exchange; RP, NP or HIAX) in combination with MALDI-TOF MS/MS and chemical/enzymatic treatments. The inset shows a comparison of MS/MS of free and pyridylaminated forms of Hex2HexNAc2Fuc1 N-glycans and exemplifies the positive effect of pyridylamination in determining the occurrence and even, based on relative intensities of the m/z 300 and 446 positive mode fragment ions, the type of core fucosylation