Abstract
Objective: This study aimed to investigate mRNA and protein expression levels of RABEX-5 and matrix metalloproteinase-9 (MMP-9), their mutual correlation, and biological behavior in gastric cancer (GC) patients. Methods: The expression levels of RABEX-5 and MMP-9 were determined by real-time quantitative PCR and Western blotting in cell lines, GC tissues, and adjacent normal tissues. In addition, RABEX-5 and MMP-9 expression was analyzed by immunohistochemistry in formalin-fixed tissues from 113 GC patients. Results: The mRNA and protein expression levels of RABEX-5 and MMP-9 in GC cell lines and GC tissues were higher than those in normal gastric mucosa cell line and adjacent normal tissues. RABEX-5 expression and MMP-9 expression in GC tissues were significantly and positively correlated. In addition, the size of tumor (p<0.001), Lauren’s classification (p=0.009), and N stage (p<0.001) were identified as the relative factors of RABEX-5 expression, whereas the expression of MMP-9 was correlated with N stage (p=0.003). The results of the multivariate analysis revealed that the independent predictive factors of overall survival were T stage (hazard ratio (HR)=2.382; p=0.028), N stage (HR=1.755; p<0.001), RABEX-5 expression (HR=0.452; p=0.004), and MMP-9 expression (HR=0.561; p=0.032). Conclusions: RABEX-5 and MMP-9 expression levels were elevated in GC tissues and were associated with tumor invasion, metastasis, and prognosis. Therefore, they may be promising prognostic indicators of survival in GC patients.
Keywords: Stomach, cancer, RABEX-5, MMP-9, prognosis
Introduction
The etiology of gastric cancer (GC) is a complex process that involves activation of oncogenes and inactivation of tumor suppressor genes at different stages, but the exact pathogenesis remains unclear [1]. Recent clinical data have demonstrated that the Ras family genes play important roles in human tumorigenesis [2]. The Rab family proteins are important for the regulation of signal transduction and cellular processes, such as differentiation, proliferation, vesicle transport, nuclear assembly, and cytoskeleton formation [3]. Ras-associated binding (Rab)-GTPases are members of the Ras family of small GTPases. RABEX-5 is a guanine nucleotide exchange factor (GEF) for RAB-5 [4]. Previous studies have reported that RABEX-5 could specifically bind to the active form of RAB-5, thereby regulating the fusion of endosomal membranes, the motility of endosomes, and intracellular signal transduction [5-6]. Numerous investigations have demonstrated that RABEX-5 plays oncogenic roles in the formation and development of malignant tumors [7]. Wang et al. [8] confirmed that silencing of RABEX-5 gene can reduce cell proliferation and colony formation, and stimulate cell apoptosis in GC cell lines.
Matrix metalloproteinases (MMPs) are a group of zinc-dependent proteins that are present in the extracellular milieu of various tissues. MMPs have been viewed as key modulators of tumor progression and metastasis [9,10]. As a member of MMPs family, MMP-9 is required for physiological processes, such as ECM remodeling during growth and development, inflammation, wound healing, angiogenesis, and leukocyte mobilization [11]. It also plays critical roles in maintaining the tumor microenvironment, which may enhance cancer cell motility and cancer growth [12]. MMP-9 gene also regulates the transcriptional level by binding of trans-activators, including AP-1 and NF-Κb, to the promoter [13]. Yoo et al. [14] reported that MMP-9 contributed to tumor metastasis via the Sonic hedgehog signaling pathway in GC.
Zhang et al. [15] showed that RABEX-5 modulated the proliferation and metastasis potential of breast cancer cells by activating the MMP-9 pathway. However the roles of RABEX-5 and MMP-9 in the development and progression of GC have not been elucidated yet. In present study, we explored the expression levels of RABEX-5 and MMP-9 in cell lines, as well as in GC and normal tissues. Furthermore, we examined the relationships between RABEX-5 or MMP-9 expression and clinicopathological characteristics. In addition, we investigated the prognostic values of RABEX-5 and MMP-9 in GC patients.
Materials and methods
Tissue samples
Patients who had histologically confirmed GC during the period from 2006 to 2010 in Tianjin Nankai Hospital, Tianjin, China, were included in the investigation. The eligibility criteria for this study included the following requirements: (1) no history of gastrectomy or other malignancy, (2) no distant metastasis or peritoneal dissemination, (3) the clinical data and the follow-up information were complete. The exclusion criteria were: (1) patients who underwent palliative surgery, (2) patients who received treatment, such as chemotherapy or radiation therapy prior to radical surgery. Furthermore, 30 fresh GC tissues and adjacent normal tissues were also collected from GC patients for analysis of the mRNA and protein expression levels of RABEX-5 and MMP-9.
Gastric cancer cell lines and normal gastric mucosa cell line
GC cell lines HGC-27, AGS, SGC7901, and MGC803 were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Human normal gastric mucosa cell line GES-1 was obtained from Biowit Technologies, Ltd. (Shenzhen, China). The HGC-27, AGS, SGC7901, MGC803, and GES-1 cell lines were incubated at 37°C in a humidified atmosphere with 5% CO2 and 95% air in RPMI 1640 medium (Thermo Electron Corporation, Beijing, China) with 10% (v/v) FBS (Life Tech, Mulgrave Victoria, Australia) and penicillin-streptomycin (10,000 IU/mL penicillin and 20 mg/mL streptomycin; Roche, Basel, Switzerland).
RNA preparation, quantitative real-time PCR
We evaluated mRNA expression levels of RABEX-5 and MMP-9 in cell lines, GC tissues and adjacent normal tissues by qRT-PCR analysis. Total RNA was extracted from frozen tissue by using TRIzol Reagent (Invitrogen, USA), following the manufacturer’s protocol. Reverse transcription was performed in a 25 mL reaction volume with 2 mg of total RNA treated with 0.5 mg of Oligo (dt), 200 U of M-MLV reverse transcriptase, 25 U of RNase inhibitor, and 2.5 mM of dNTP to synthesize the first-strand cDNA (Promega, USA), following the manufacturer’s recommendations. The reaction system was incubated at 70°C for 5 min (primer annealing) and 42°C for 1 h (synthesis), and resulting cDNA was stored at -20°C. The resulting cDNA was then subjected to qRT-PCR for evaluation of the relative mRNA levels of RABEX-5, MMP-9 and GAPDG (as internal control) with the following primers: (forward/reverse sequences) RABEX-5 (5’-TTGGACAGATGGAATTGCAA/GTTGCAGTGGTGGAGGAAGT-3’), MMP-9 (5’-AGGACGGCAATGCTGATG/GTGGTGGCGGTTGATGCT-3’)and GAPDH (5’-GAAGGTGAAGGTCGGAGTC/GAAGATGGTGATGGGATTTC-3’). Gene special amplification was performed in an ABI PRISM 7900HT real-time PCR system (Life Technologies, USA) with a 20 μL PCR mix containing 2 μL of cDNA, 10 μL of 2×SYBR Green PCR Master Mix (Invitrogen, USA), and 200 nM of the appropriate primers. The mixture was preheated for 5 min at 95°C, followed by 30 cycles of amplification (30 s at 94°C, 30 s at 48°C, and 1 min at 72°C), and a final elongation step of 72°C for 10 min. The relative quantification of RABEX-5 and MMP-9 mRNA expression were normalized to GAPDH value (2-ΔΔCT method). Experiments were repeated three times.
Western blotting
RABEX-5 and MMP-9 protein expression levels were also assessed by Western blotting.Total protein extracts were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel electrophoresis and were electrotransferred to PVDF membranes. After blocking nonspecific binding sites for 60 min with 5% nonfat milk, the membranes were incubated overnight at 4°C with a primary rabbit antihuman RABEX-5 (ab113480, 1:1,000 dilution; abcam) and rabbit antihuman MMP-9 polyclonal antibody (ab76003, 1:1,000 dilution, abcam). The membranes were then washed 3×15 min with PBS-T and probed with a horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin antibodies (1:2,000 dilution; ZhongShan Biotechnology) for 60 min at room temperature. The membranes were then washed three times with PBS-T for 10 min. The immunocomplexes was visualized by enhanced chemoluminescence system (Cell Signaling, USA). The intensity of the protein bands was determined by densitometry using AlphaEaseFC software (Alpha Innotech, USA). To confirm equal loading, GAPDH antibody was served as a loading control.
Immunohistochemistry
Paraffin blocks of tumors were cut into 4-mm slices and mounted on saline-coated slides. Sections were deparaffinized in xylene and rehydrated in graded alcohols. Antigen retrieval was performed by immersing the sections in 10 mM sodium citrate buffer (citric acid and sodium citrate, pH 6.0) for 20 minutes at 98°C in a water bath. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide in water. Sections were incubated with rabbit anti-RABEX-5 (ab113480, Abcam) and anti-MMP-9 (ab76003, Abcam) antibodies at a dilution of 1:50 at 4°C overnight. The sections were then washed three times with phosphate-buffered saline (PBS) and incubated with the corresponding secondary antibodies for 30 min at 37°C, after which the sections were washed with PBS and incubated for 1 min with 3,30-diaminobenzidine (DAB). The sections were then counterstained with hematoxylin, dehydrated, cleared, and permanently mounted with resinous mounting medium. All the procedures were carried out at room temperature. For negative controls, PBS was used in place of the primary antibody. All sections were assessed blindly by two independent observers, and in case of assessing disagreement, a third independent assessment was performed. The staining score in GC cells in each slide was assessed according to the staining intensity and the percentage of the positive cells. The staining intensity was scored as 0 (negative), 1 (very weak), 2 (weak), 3 (medium), and 4 (strong). The extent of staining was scored as 0 (0-10%), 1 (10%-30%), 2 (30%-50%), 3 (50%-75%), and 4 (>75%) according to the percentage of positive-staining cells in relation to the total cancer cells. The expression levels of RABEX-5 and MMP-9 in each slide were scored as the sum of intensity and extent of positive-staining cells. The slide with a final staining score of more than 3 was defined as positive expression [16].
Statistical analysis
A paired sample t-test was utilized to compare continuous variables. The associations between the expression rates of proteins and clinicopathological variables were analyzed using the chi-square test. Survival analysis was performed by the Kaplan-Meier method and compared by the log-rank test. Multivariate analysis was conducted to find the potential independent prognostic factors in GC. All statistical calculations were performed using SPSS Statistics 17 software (SPSS, Chicago, IL, USA). Significance was defined as p-value <0.05.
Results
mRNA and protein expression values of RABEX-5 and MMP-9 in cell lines, GC tissues, and adjacent normal tissues
RABEX-5 and MMP-9 mRNA and protein expression values were respectively determined in GC cell lines, normal gastric mucosa cell line, GC tissues and their adjacent normal tissues. RABEX-5 and MMP-9 mRNA expression levels in GC cell lines HGC-27 (0.39±0.14; 0.25±0.12), AGS (0.23±0.11; 0.20±0.09), SGC7901 (0.21±0.06; 0.33±0.14), and MGC803 (0.25±0.11; 0.18±0.06) were significantly higher than those in the normal gastric mucosa cell line GES-1 (0.08±0.04; 0.06±0.07). The relative mRNA expression values of RABEX-5 and MMP-9 in GC tissues were more elevated than those in the adjacent normal tissues (RABEX-5, 0.32±0.10 VS 0.07±0.04, p<0.001; MMP-9, 0.23±0.11 VS 0.09±0.05, p<0.001) (Figure 1).
Figure 1.
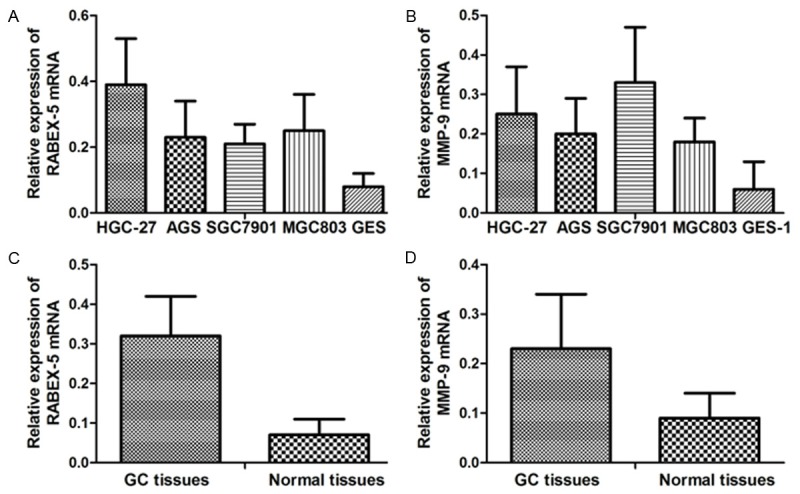
A. Relative RABEX-5 mRNA expression values in cell lines; B. Relative MMP-9 mRNA expression values in cell lines; C. Relative RABEX-5 mRNA expression values in GC tissues and adjacent normal tissues; D. Relative MMP-9 mRNA expression values in GC tissues and adjacent normal tissues.
RABEX-5 and MMP-9 protein expression levels in GC cell lines HGC-27 (1.21±0.54; 1.35±0.42), AGS (1.23±0.40; 1.17±0.45), SGC7901 (0.98±0.42; 1.01±0.52), and MGC803 (1.27±0.51; 1.02±0.46) were higher than those in GES-1 (0.48±0.24; 0.55±0.17). The relative protein expression levels of RABEX-5 and MMP-9 in GC tissues were significantly more augmented than those in the adjacent normal tissues (RABEX-5, 1.37±0.57 VS 0.51±0.22, p<0.001; MMP-9, 1.21±0.44 VS 0.45±0.37, p<0.001) (Figure 2).
Figure 2.
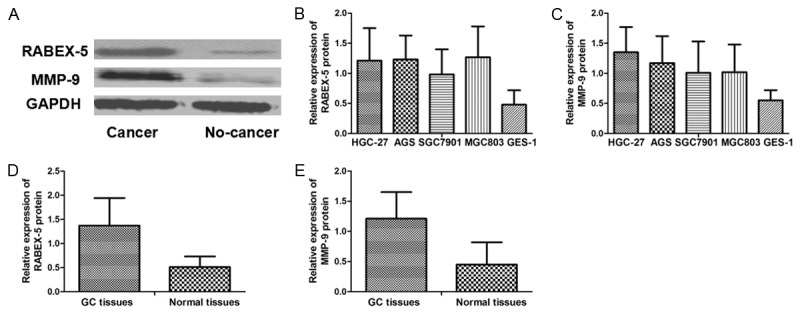
A. Western blot analysis of RABEX-5 and MMP-9 protein; B. Relative RABEX-5 protein expression values in cell lines; C. Relative MMP-9 protein expression values in cell lines; D. Relative RABEX-5 protein expression values in GC tissues and adjacent normal tissues; E. Relative MMP-9 protein expression values in GC tissues and adjacent normal tissues.
Patient characteristics
Based on inclusion and exclusion criteria, a total of 113 GC patients were eligible to participate in this study. The 5-year survival rate (5-YSR) of GC patients was 43.0%. The other clinicopathological characteristics of patients are shown in Table 1.
Table 1.
Clinicopathological characteristics of GC patients
| Variables | Cases (%) |
|---|---|
| Gender | |
| Male | 69 (61.1) |
| Female | 44 (38.9) |
| Age (years) | |
| ≤60 | 73 (64.6) |
| >60 | 40 (35.4) |
| Size of tumor (cm) | |
| ≤5 | 74 (65.5) |
| >5 | 39 (34.5) |
| Location of tumor | |
| Lower 1/3 | 43 (38.1) |
| Middle 1/3 | 27 (23.9) |
| Upper 1/3 | 43 (38.1) |
| Degree of differentiation | |
| Well/Moderate | 11 (9.7) |
| Poor | 102 (90.3) |
| Lauren’s classification | |
| Intestinal type | 73 (64.6) |
| Diffuse type | 40 (35.4) |
| T stage* | |
| T1 | 8 (7.1) |
| T2 | 4 (3.5) |
| T3 | 9 (8.0) |
| T4 | 92 (81.4) |
| N stage* | |
| N0 | 38 (33.6) |
| N1 | 27 (23.9) |
| N2 | 21 (18.6) |
| N3 | 27 (23.9) |
| RABEX-5 expression | |
| Negative | 61 (54.0) |
| Positive | 52 (46.0) |
| MMP-9 expression | |
| Negative | 65 (57.5) |
| Positive | 48 (42.5) |
7th TNM classification.
Correlation between RABEX-5 and MMP-9 expression in GC tissue
We analyzed the correlation between RABEX-5 expression and MMP-9 expression in GC tissues (by immunohistochemical staining). RABEX-5 and MMP-9 were localized mainly in the cytoplasm of GC cells (Figure 3). In the present study, there were 52 RABEX-5 5-positive and 48 MMP-9 9-positive expression patients. A positive correlation was found between RABEX-5 expression and MMP-9 expression in GC tissues (R=0.284, p=0.003).
Figure 3.
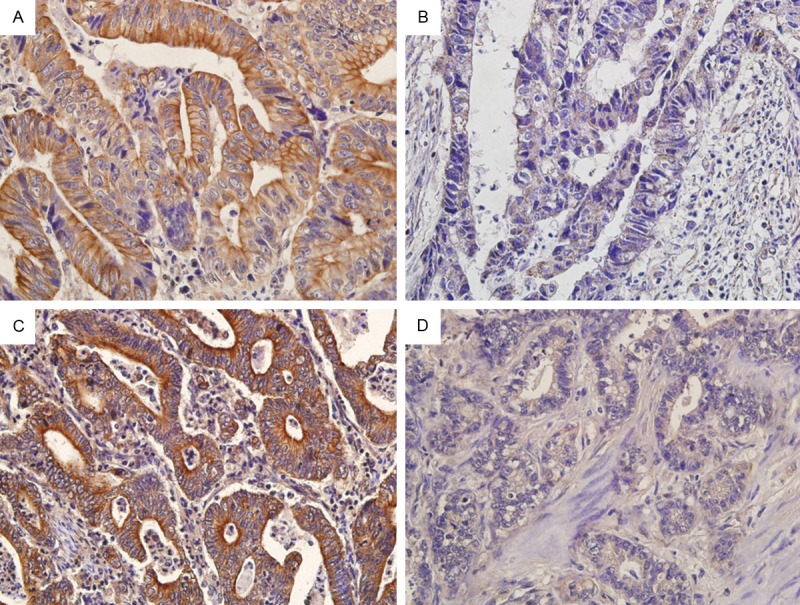
A. RABEX-5 was positively expressed in GC tissues; B. RABEX-5 was negatively expressed in GC tissues; C. MMP-9 was positively expressed in GC tissues; D. MMP-9 was negatively expressed in GC tissues. Magnification=400×(a, b, c and d).
Relationships between RABEX-5 or MMP-9 expression in GC tissues and clinicopathological variables
RABEX-5 and MMP-9 expression levels in patients with different different clinical characteristics, such as gender, ages, size of tumor, location of tumor, degree of differentiation, Lauren’s classification, T- and N-stages, were analyzed. Ultimately, the size of tumor (p<0.001), Lauren’s classification (p=0.009), and N stage (p<0.001) were identified as the relative factors of RABEX-5 expression, whereas the N stage (p=0.003) was recognized as the relative factors of MMP-9 expression in GC tissues (Table 2).
Table 2.
Relationships between RABEX-5 or MMP-9 expression in GC tissues and clinicopathological variables
| Variables | RABEX-5-positive cases | Univariate p value | MMP-9-positive cases | Univariate p value |
|---|---|---|---|---|
| Gender | 0.100 | 0.904 | ||
| Male | 36 | 29 | ||
| Female | 16 | 19 | ||
| Age | 0.815 | 0.997 | ||
| ≤60 | 33 | 31 | ||
| >60 | 19 | 17 | ||
| Size of tumor | <0.001 | 0.169 | ||
| ≤5 | 25 | 28 | ||
| >5 | 27 | 20 | ||
| Location of tumor | 0.539 | 0.437 | ||
| Lower 1/3 | 17 | 15 | ||
| Middle 1/3 | 13 | 13 | ||
| Upper 1/3 | 22 | 20 | ||
| Degree of differentiation | 0.499 | 0.283 | ||
| Well/Moderate | 4 | 3 | ||
| Poor | 48 | 45 | ||
| Lauren’s classification | 0.009 | 0.111 | ||
| Intestinal type | 27 | 27 | ||
| Diffuse type | 25 | 21 | ||
| T stage* | 0.090 | 0.110 | ||
| T1 | 2 | 1 | ||
| T2 | 0 | 1 | ||
| T3 | 6 | 2 | ||
| T4 | 44 | 44 | ||
| N stage* | <0.001 | 0.003 | ||
| N0 | 9 | 8 | ||
| N1 | 10 | 12 | ||
| N2 | 14 | 10 | ||
| N3 | 19 | 18 |
7th TNM classification.
Analysis of survival in GC patients
Univariate analysis showed that the location of tumor, degree of differentiation, T stage, N stage, RABEX-5 expression, and MMP-9 expression were prognostic factors for overall survival (OS). Patients with RABEX-5 5-positive expression or MMP-9 9-positive expression had a poorer OS than patients with RABEX-5 5-negative expression or MMP-9 9-negative expression (Figure 4). Furthermore, the Cox proportional hazard regression model was used to find the potential independent prognostic factors in GC. Finally, T stage (hazard ratio (HR)=2.382; p=0.028), N stage (HR=1.755; p<0.001), RABEX-5 expression (HR=0.452; p=0.004), and MMP-9 expression (HR=0.561; p=0.032) were identified as the independent factors of the OS (Table 3).
Figure 4.
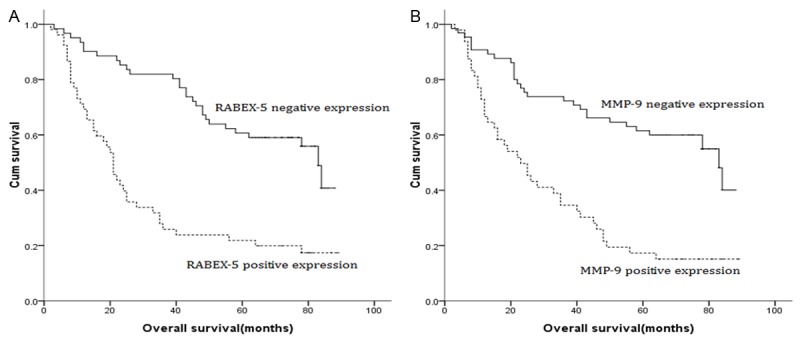
A. Survival curve of GC patients according to RABEX-5 expression (negative or positive); B. Survival curve of GC patients according to MMP-9 expression (negative or positive).
Table 3.
Survival analysis of GC patients
| Variables | 5-YSR (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
|
|||||
| χ2 value | p value | HR value | p value | ||
| Gender | 0.163 | 0.686 | |||
| Male | 42.0 | ||||
| Female | 44.5 | ||||
| Age | 0.324 | 0.569 | |||
| ≤60 | 43.2 | ||||
| >60 | 42.5 | ||||
| Size of tumor | 5.889 | 0.015 | |||
| ≤5 | 50.0 | ||||
| >5 | 29.3 | ||||
| Location of tumor | 3.354 | 0.187 | |||
| Lower 1/3 | 53.5 | ||||
| Middle 1/3 | 46.6 | ||||
| Upper 1/3 | 30.2 | ||||
| Degree of differentiation | 4.564 | 0.033 | |||
| Well/Moderate | 72.7 | ||||
| Poor | 39.4 | ||||
| Lauren’s classification | 0.016 | 0.898 | |||
| Intestinal type | 43.8 | ||||
| Diffuse type | 41.4 | ||||
| T stage* | 15.693 | 0.001 | 2.382 | 0.028 | |
| T1 | 100.0 | ||||
| T2 | 75.0 | ||||
| T3 | 66.7 | ||||
| T4 | 34.8 | ||||
| N stage* | 54.572 | <0.001 | 1.755 | <0.001 | |
| N0 | 71.1 | ||||
| N1 | 48.1 | ||||
| N2 | 30.4 | ||||
| N3 | 7.4 | ||||
| RABEX-5 expression | 25.699 | <0.001 | 0.452 | 0.004 | |
| Negative | 60.7 | ||||
| Positive | 21.9 | ||||
| MMP-9 expression | 23.423 | <0.001 | 0.561 | 0.032 | |
| Negative | 63.1 | ||||
| Positive | 19.4 | ||||
7th TNM classification.
Discussion
GC is one of the most common causes of death worldwide [17]. During the past decades, the introduction of advanced diagnostic technologies and the common use of multimodal therapy (chemotherapy, radiation therapy, and surgery) have improved patient satisfaction and outcomes. However, the long-term survival rate is still low [18]. Moreover, the molecular mechanisms underlying the development of GC remain incompletely understood. Therefore, the exploration of promising novel predictive factors is urgently needed to improve the prognosis of GC.
Similarly to other small GTPases, RAB-5 is activated by an exchange of bound GDP with GTP. RABEX-5 was identified as an interactor of Rabaptin-5 that possesses GEF activity toward RAB-5 and related GTPases [19]. Upregulation of RABEX-5 has been detected in various cancer types, including breast cancer, prostate cancer, colorectal cancer, gastric cancer, and lung cancer [20]. RABEX-5 acts as an oncogene and due to is its involvement in the formation and development of malignant tumors, it might influence tumor biological behavior. Wang et al. [8] reported that RABEX-5 expression was elevated in GC tissues, and the knockdown of RABEX-5 gene inhibited the wound healing, migration, and invasive abilities of GC cells. Furthermore, the authors found that RABEX-5 expression was associated with tumor size and lymph node metastasis. Zhang et al. [21] demonstrated that the positive RABEX-5 expression was correlated with tumor recurrence in non-small-cell lung cancer. In breast cancer, it was reported that positive RABEX-5 expression was associated with axillary lymph node metastasis, and RABEX-5 silencing reduced cancer cell proliferation, colony formation, and migration ability in vitro [15]. Zhang et al. [22] evidenced that the level of RABEX-5 mRNA was elevated in prostate cancer patients, and high RABEX-5 mRNA expression was a predictor of poor prognosis. In the current study, the mRNA and protein expression levels of RABEX-5 in GC cell lines and tissues were both higher than those in normal gastric mucosa cell line and adjacent non-tumor tissues. The positive RABEX-5 expression was correlated with a larger tumor size, diffuse type, and advanced N stage, which indicated that RABEX-5 was a key participant in the carcinogenesis of GC.
In a previous study, MMP-9 played an important role in tumorigenesis and metastasis formation. MMP-9 degraded predominantly gelatin and type IV, V, XI, and XVI collagen, a major component of the ECM and basement membrane, which appeared to be exceedingly crucial in tumor cell invasion and metastasis [23].High expression of MMP-9 was associated with poor prognosis in various tumors. Sampieri et al. [24] demonstrated that MMP9 overexpression exerted important influence in cancer invasion, which was associated with metastasis and unfavorable prognosis in GC. Zeng et al. [25] reported that the overexpression of MMP-9 was related to tumor aggressiveness in colorectal cancer. Lee et al. [26] found MMP-9 expression was associated with poor outcomes in patients with lung cancer; furthermore, they demonstrated that MMP-9 expression was identified as an independent predictor of relapse. Chu et al. [27] reported that MMP-9 was correlated with the depth of invasion, lymph node metastasis, and distant metastasis in GC, and they confirmed that MMP-9 was an independent prognostic factor for both disease-free and overall survival. In the current study, significant differences in MMP-9 mRNA and protein expression were established between GC cell lines or tissues and normal gastric mucosa cell line or adjacent non-tumor tissues. Our results also revealed that significant correlations existed between MMP-9 expression and N stage. We demonstrated that MMP-9 was aberrantly activated in GC tissue, suggesting that the overexpression of MMP-9 may promote the invasive behavior and the metastasis processes in GC.
To assess the prognostic value of RABEX-5 and MMP-9 expression levels in GC, we analyzed the expression of proteins in patients with GC by using the OS values as an indicator. The Kaplan-Meier curves obtained confirmed that patients with RABEX-5- or MMP-9-positive expression had poorer OS than those with RABEX-5-5 or MMP-9-negative expression. In addition to T stage and N stage, the results of the multivariate Cox regression analysis identified also RABEX-5 expression and MMP-9 expression as independent predictors of the survival of GC patients. Determination of RABEX-5 and MMP-9 expression levels may facilitate the identification of high-risk GC patients and thus aid the selection of appropriate therapy options. RABEX-5 and MMP-9, which could be potential therapeutic targets, should be considered as prognostic indicators of poor survival in GC.
A novel finding of the present study was the correlation between RABEX-5 expression and MMP-9 expression in GC. RABEX-5 was reported to induce MMP-9 expression in human tumor models [15]. In this investigation, we found that there was a positive correlation between the protein expression of RABEX-5 and MMP-9 in GC tissues. We deduced that RABEX-5 and MMP-9 affected each other in the carcinogenesis and tumor progression of GC.
This study had several limitations. We utilized a relatively small sample size of patients, which limited the level of evidence. Also, we did not obtain sufficient data to elucidate the molecular mechanism of these processes. Accordingly, further investigations with a larger sample size that explore the mechanisms of the relationship between RABEX-5 and MMP-9 expression should be conducted in the future.
In this examination, we evidenced that both RABEX-5 and MMP-9 were upregulated in GC, the expression of RABEX-5 was positively correlated with that of MMP-9 in GC, and RABEX-5 and MMP-9 influenced the prognosis of GC.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.31470816).
Disclosure of conflict of interest
None.
References
- 1.Oh JH, Jung SH, Hong SJ, Rhyu MG. DNA Methylation as Surrogate Marker For Gastric Cancer. J Cancer Prev. 2015;20:172–178. doi: 10.15430/JCP.2015.20.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenring JR, Nam KT. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer. 2011;104:33–36. doi: 10.1038/sj.bjc.6605983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg-Larsen A, Landsverk OJ, Progida C, Gregers TF, Bakke O. Differential regulation of Rab GTPase expression in monocyte-derived dendritic cells upon lipopolysaccharide activation: a correlation to maturation-dependent functional properties. PLoS One. 2013;8:e73538. doi: 10.1371/journal.pone.0073538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 5.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22:461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiuchi H, Lippé R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effectorrecruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Zhang M, Hu G, Cai Q, Xu T. Elevated RABEX-5 protein expression predicts poor prognosis in combined small cell lung cancer. Tumour Biol. 2015;36:8287–8293. doi: 10.1007/s13277-015-3562-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Lu A, Chen X, Wei L, Ding J. RABEX-5 is upregulated and plays an oncogenic role in gastric cancer development by activating the VEGF signaling pathway. PLoS One. 2014;9:e113891. doi: 10.1371/journal.pone.0113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymaticdegradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 10.Curran S, Murray GI. Matrix metalloprteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Zhao Y, Lu C, Fu M, Dou T, Tan X. Signatures of positive selection at hemopexin (PEX) domain of matrix metalloproteinase-9 (MMP-9) gene. J Biosci. 2015;40:885–890. doi: 10.1007/s12038-015-9577-6. [DOI] [PubMed] [Google Scholar]
- 12.Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis and angiogenesis. Surg Oncol Clin N Am. 2001;10:383–392. [PubMed] [Google Scholar]
- 13.Tamatani T, Azuma M, Ashida Y, Motegi K, Takashima R, Harada K, Kawaguchi S, Sato M. Enhanced radiosensitization and chemosensitization in NF-kappaBsuppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108:912–921. doi: 10.1002/ijc.11640. [DOI] [PubMed] [Google Scholar]
- 14.Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Min J, Wang Y, Li Y, Li H, Liu Q, Liang X, Mu P, Li H. RABEX-5 plays an oncogenic role in breast cancer by activating MMP-9 pathway. J Exp Clin Cancer Res. 2013;32:52. doi: 10.1186/1756-9966-32-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Liu H, Luo X, Deng J, Pan Y, Liang H. Overexpression of SMYD3 and matrix metalloproteinase-9 are associated with poor prognosis of patients with gastric cancer. Tumour Biol. 2015;36:4377–4386. doi: 10.1007/s13277-015-3077-z. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Liu Y, Kong F, Xin W, Li X, Liang H, Jia Y. Elevated Levels of SET and MYND Domain-Containing Protein 3 Are Correlated with Overexpression of Transforming Growth Factor-β1 in Gastric Cancer. J Am Coll Surg. 2015;221:579–590. doi: 10.1016/j.jamcollsurg.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H, Jahanshahi M, Horvath EA, Liu HY, Pfleger CM. Rabex-5 ubiquitin ligase activity restricts Ras signaling to establish pathway homeostasis in Drosophila. Curr Biol. 2010;20:1378–1382. doi: 10.1016/j.cub.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Jia Y, Kong F, Hu G, Cai Q, Xu T. Elevated RABEX-5 expression predicts poor prognosis in non-small-cell lung cancer. Am J Cancer Res. 2015;5:2849–2855. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Cheng S, Wang A, Ma H, Yao B, Qi C, Liu R, Qi S, Xu Y. Expression of RABEX-5 and its clinical significance in prostate cancer. J Exp Clin Cancer Res. 2014;33:31. doi: 10.1186/1756-9966-33-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res. 2004;10:8229–8234. doi: 10.1158/1078-0432.CCR-04-0424. [DOI] [PubMed] [Google Scholar]
- 24.Sampieri CL, de la Peña S, Ochoa-Lara M, Zenteno-Cuevas R, León-Córdoba K. Expression of matrix metalloproteinases 2 and 9 in human gastric cancer and superficial Gastritis. World J Gastroenterol. 2010;16:1500–1505. doi: 10.3748/wjg.v16.i12.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20:749–755. doi: 10.1093/carcin/20.5.749. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, Shim HS, Lee S, Lee JG, Kim DJ, Chung KY. Prognostic effect of matrix metalloproteinase-9 in patients with resected Non small cell lung cancer. J Cardiothorac Surg. 2015;10:44. doi: 10.1186/s13019-015-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu D, Zhang Z, Li Y, Zheng J, Dong G, Wang W, Ji G. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer. 2011;129:887–95. doi: 10.1002/ijc.25734. [DOI] [PubMed] [Google Scholar]


