Key Clinical Message
A high index of suspicion is needed when a patient presents with thyroid mass in the settings of an advanced CRC. Secondary thyroid malignancy should be considered unless proven otherwise. reatment should be determined considering extent of CRC metastasis, patient's general condition, and presence of local symptoms.
Keywords: Colorectal cancer, HER2/neu, metastasis, molecular characteristics, thyroid
Introduction
Colorectal cancer (CRC) is a major cause of morbidity and mortality worldwide. Despite advances in screening procedures and the use of adjuvant therapy, up to 50% of patients will eventually develop metastatic disease, most frequently to the lymph nodes, liver and lungs, even after curative resection of primary tumor 1. The thyroid gland is considered an unusual site for CRC metastasis, although the precise incidence is unclear and may vary widely depending on the analyzed study population. While thyroid metastasis from CRC and other malignancies are rarely reported in clinical series 2, autopsy studies have shown that microscopic metastasis may be found in up to 24% of cases with widespread malignant diseases 3. Similar to the initial phase of primary thyroid neoplasms, the metastatic tumors may remain asymptomatic until advanced stages have been reached and local symptoms develop. However, once thyroid metastasis is detected, patients' prognosis is considered poor, with a life expectancy of few months. Behavior of thyroid metastasis from CRC is considered more aggressive than metastases from the kidney or breast and it is usually associated with advanced progressive disease refractory to adjuvant therapies 3, 4.
We report a case of thyroid metastasis from an advanced, aggressive adenocarcinoma of the colon with a comprehensive molecular characterization of both tumor sites.
Case presentation
A 42‐year‐old woman was found to have an asymptomatic thyroid mass on routine follow‐up examination by her primary care physician. Ultrasound imaging (US) of the thyroid showed a 0.9 cm left thyroid lobe mass. Fine‐needle aspiration (FNA) biopsy revealed atypical cells of undetermined significance; however, further intervention was not pursued. Her past medical history was significant for Type I Chiari Malformation, hypothyroidism, Celiac disease, and stage IV adenocarcinoma of the colon that was found during work up for worsening abdominal pain. Computer tomography (CT) of the abdomen and pelvis at that time revealed an 8.5 cm mass in the cecum with liver lesions and enlarged lymph nodes highly suspicious for metastatic disease. The patient did not report weight loss or rectal bleeding.
Diagnosis was confirmed by colonoscopy and biopsy of the tumor revealing exophytic adenocarcinoma of the cecum with mucinous histopathological features. Staging workup including fluoro‐2‐deoxy‐D‐glucose positron emission tomography (FDG‐PET) scan revealed intense FDG uptake in the cecum, ascending colon, locoregional lymph nodes and right lobe of the liver consistent with widespread metastatic disease. There was no enhanced FDG uptake in the thyroid at that time.
The patient underwent a right hemicolectomy and partial hepatectomy. Surgical pathology revealed a moderately differentiated adenocarcinoma of the cecum with a desmoplastic phenotype on a mucinous background and tumor metastasis in 3 of 19 resected lymph nodes (Fig. 1A). The hepatic lesions were found to be colon cancer metastases (not shown).
Figure 1.
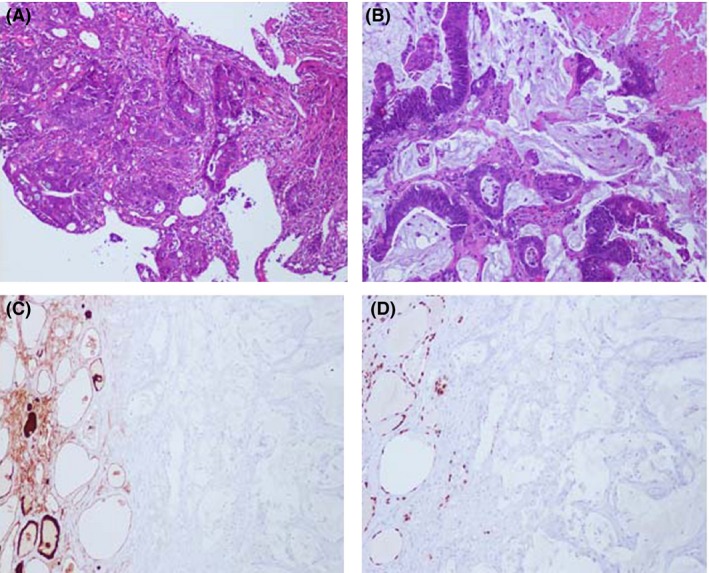
Histological and immunohistological characterization of thyroid metastasis. (A) H&E of moderately differentiated primary tumor with mucinous features; (B) H&E of metastatic thyroid lesion reveals poorly differentiated colonic adenocarcinoma with desmoplastic features in a background of extracellular mucin; (C) Immunohistochemistry for thyroglobulin (TG) demonstrates staining of the resident thyroid tissue (brown) and no staining of metastatic tumor cells; (D) Immunohistochemistry for thyroid transcription factor‐1 (TTF‐1) demonstrates staining of the resident thyroid tissue (brown) and no staining of metastatic tumor cells.
Multiple adjuvant therapy regiments were tried, including FOLFOX (5‐FU, leucovorin, oxaliplatin), FOLFIRI (5‐FU, leucovorin, oxaliplatin, Irinotecan)/Bevacizumab, 5‐Azacytidine, histone deacetylase inhibitor (Romidepsin), Niemann‐Pick type C monoclonal antibody (Ensituximab), and Bromodomain‐containing protein 4 (BRD4) inhibitor, but her disease progressed with lung metastases discovered on the follow‐up imaging.
Two years after her colon cancer surgery and one year after the incidental finding of an asymptomatic left thyroid lobe mass, the patient started experiencing dysphagia and neck discomfort. A repeat US of the thyroid showed a significant increase in size of the left thyroid lobe mass from 0.9 to 4.2 cm extending to the isthmus and right lobe. A repeat FNA biopsy of the mass revealed a malignant neoplasm consistent with colonic origin (Fig. 1B). The metastatic nature of the tumor was confirmed by immunohistochemical staining positive for the GI markers CDX2 and CK20 (not shown).
Because of the significant thyroid metastasis growth with worsening compressive symptoms, the patient underwent a total thyroidectomy. Surgical pathology demonstrated multicentric metastatic colorectal adenocarcinoma with a dominant left thyroid lobe mass and extensive marginal involvement.
Because multiple prior adjuvant chemotherapy regiments failed to control the progression of her disease, the patient was referred to the National Cancer Institute‐National Institutes of Health and screened for inclusion of a phase I clinical trial evaluating therapeutic, autologous transduced dendritic cell vaccine expressing HER2/neu (http://www.clinicaltrials.gov/ct2/show/NCT01730118). To confirm the absence of two distinct primary tumors (an exclusion criterion for the trial), the colonic origin of the thyroid mass was validated using H&E and immunohistochemistry by staining of archived tissue for the thyroid‐specific markers thyroglobulin (Tg) and thyroid transcription factor‐1 (TTF‐1) (Fig. 1B). Since the primary targets of this vaccine are neoplastic cells that express HER2/neu, we stained both tumor sites for HER2/neu and quantified expression according to the American Society of Clinical Oncology/College of American Pathologists guidelines 5. A stronger surface expression of HER2/neu in the metastatic site compared to the primary tumor was noted (Fig. 2 A and B). The expression of HER2/neu in the colon and thyroid metastasis was characterized as 2+ (moderate) and 3+ (high), respectively.
Figure 2.
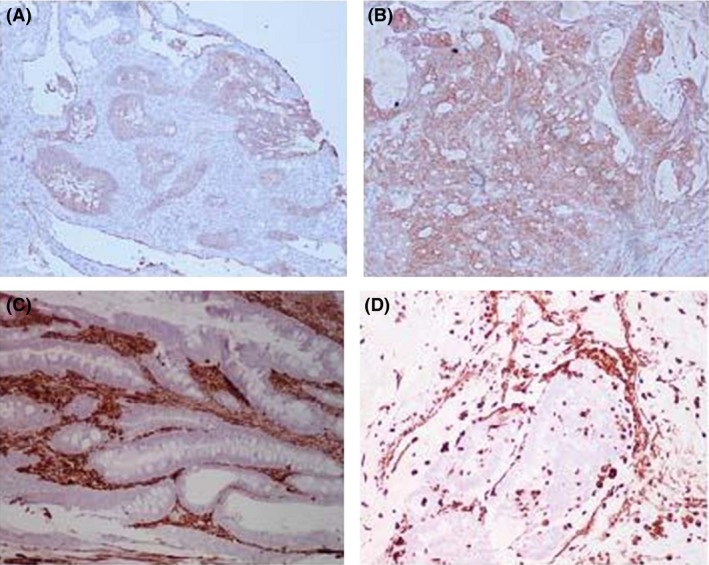
Immunohistochemistry for HER2/neu and vimentin of the primary and metastatic colon cancer sites. (A) Immunohistochemistry for HER2/neu of primary colon tumor revealing weak to moderately complete membrane staining in >10% of tumor cells; (B) Immunohistochemistry for HER2/neu of thyroid metastasis revealing strong, complete membrane staining in >10% of tumor cells; (C) Immunohistochemistry for vimentin in the primary colon tumor shows negative staining of the tumor cells with strong stromal tissue staining (brown); (D) Immunohistochemistry for vimentin in the thyroid metastasis shows negative staining of the tumor cells with strong stromal tissue staining (brown).
To gain deeper insights into this metastatic event and compare any possible trends to the pattern of differential expression of HER2/neu between the tumor sites, the expression of vimentin, a marker of epithelial‐to‐mesenchymal transition implicated in metastasis, was assessed in the primary and metastatic site (Fig. 2 C and D). Previous studies have demonstrated a unique phenomenon of vimentin gene hypermethylation and underexpression in colorectal cancer with methylation status correlating with a higher Duke's stage and the presence of liver metastases 6, 7. In this case, both the colon and thyroid specimens were negative for vimentin while the surrounding stromal tissue demonstrated strong staining (Fig. 2 C and D). An informed consent was obtained, and the patient was enrolled in therapy with HER2/neu vaccine.
Discussion
Despite the fact that the thyroid gland is one of the most vascularized organs of the body, clinically evident metastases to the thyroid are extremely rare 2, 8. While the reasons for this phenomenon have not been completely understood, it has been proposed that the high blood velocity and oxygen consumption dynamics of the thyroid may promote an inhospitable environment for metastatic seeding 9.
The development of a thyroid nodule in a patient with a known prior malignancy may pose a significant diagnostic and management challenge. Carcinomas that frequently metastasize to the thyroid gland include breast, lung, and kidney, while metastasis from CRC is considered extremely unusual 2, 10.
Due to the rarity of thyroid CRC metastases, the literature deals exclusively with case reports. Fifty‐two cases have been published since 1931. The metastatic involvement of the thyroid gland does not seem to show gender or age predilection. The mean time from CRC diagnosis to thyroid metastasis was 48 months. Other commonly involved metastatic sites included liver, lungs, and lymph nodes.
The presentation of CRC thyroid metastases is variable and nonspecific. The most common presenting symptoms were a palpable neck mass, dysphagia, and hoarseness. FNA of a suspicious thyroid mass usually provides a rapid and definitive diagnosis. One should bear in mind, however, that the diagnosis, especially at its initial stages, can be very confusing and results from the US and FNA can be misleading. In situations like this, additional test such as histopathological evaluation and immunohistochemical staining for specific biomarkers can be very helpful. The primary versus secondary nature of a thyroid neoplasm can be assessed by immunohistochemical staining for the thyroid specific markers TTF‐1 and Tg. Immunostaining for cellular keratins CK20 and CK7 or transcription factor CDX2 has been employed for confirming the GI etiology of the CRC metastatic tumors 11, 12. To our knowledge, this is the first report to frame the metastasis of colon cancer to the thyroid gland in the context of differential HER2/neu expression. Our observations make it tempting to hypothesize an underlying link of HER2/neu directed metastasis to the thyroid from a primary colonic tumor, but pathologic stains alone cannot establish a causative association. In addition, there is no clear consensus on the role of differential HER2/neu expression in metastasis, and very few papers have characterized differential HER2/neu status in metastasis outside of the breast cancer 13, 14.
Treatment options included thyroidectomy or thyroid lobectomy with or without adjuvant chemotherapy, chemotherapy alone, and radiotherapy. There was no survival benefit of operative versus nonoperative management of CRC thyroid metastases with a median survival of 8 months.
Conclusion
A high index of suspicion is needed when a patient presents with thyroid mass in the settings of an advanced, progressive CRC, and secondary thyroid malignancy should be considered unless proven otherwise. The final treatment strategy should be determined considering the extent of metastasis to other organs, the patient's general condition, and the presence or absence of local symptoms. With the growing accessibility of tumor profiling at the genetic and immunohistological level and the development of new therapeutic strategies, the prognosis and treatment decisions in the future will increasingly rely on molecular characterizations of underlying pathological mechanisms.
Informed Consent
An informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal on request.
Conflict of Interest
None declared.
References
- 1. Kindler, H. L. , and Shulman K. L.. 2001. Metastatic colorectal cancer. Curr. Treat. Options Oncol. 2:459–471. [DOI] [PubMed] [Google Scholar]
- 2. Papi, G. , Fadda G., Corsello S. M., Corrado S., Rossi E. D., Radighieri E., et al. 2007. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10‐year experience. Clin. Endocrinol. (Oxf) 66:565–571. [DOI] [PubMed] [Google Scholar]
- 3. Shimaoka, K. , Sokal J. E., and Pickren J. W.. 1962. Metastatic neoplasms in the thyroid gland. Pathological and clinical findings. Cancer 15:557–565. [DOI] [PubMed] [Google Scholar]
- 4. Calzolari, F. , Sartori P. V., Talarico C., Parmeggiani D., Beretta E., Pezzullo L., et al. 2008. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 28(5B):2885–2888. [PubMed] [Google Scholar]
- 5. Wolff, A. C. , Hammond M. E., Schwartz J. N., Hagerty K. L., Allred D. C., Cote R. J., et al. 2007. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25:118–145. [DOI] [PubMed] [Google Scholar]
- 6. Zou, H. , Harrington J. J., Shire A. M., Rego R. L., Wang L., Campbell M. E., et al. 2007. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol. Biomark. Prev. 16:2686–2696. [DOI] [PubMed] [Google Scholar]
- 7. Shirahata, A. , Sakata M., Sakuraba K., Goto T., Mizukami H., Saito M., et al. 2009. Vimentin methylation as a marker for advanced colorectal carcinoma. Anticancer Res. 29:279–281. [PubMed] [Google Scholar]
- 8. Ferrozzi, F. , Bova D., Campodonico F., De Chiara F., Conti G. M., and Bassi P.. 1998. US and CT findings of secondary neoplasms of the thyroid–a pictorial essay. Clin. Imaging 22:157–161. [DOI] [PubMed] [Google Scholar]
- 9. Mirallie, E. , Rigaud J., Mathonnet M., Gibelin H., Regenet N., Hamy A., et al. 2005. Management and prognosis of metastases to the thyroid gland. J. Am. Coll. Surg. 200:203–207. [DOI] [PubMed] [Google Scholar]
- 10. Goatman, C. , Goldsmith P. J., Antonopoulos V., and Ali B.. 2012. Metastasis of colorectal adenocarcinoma to the thyroid: a case report and review of the literature. Case Rep. Surg. 2012:179407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips, J. S. , Lishman S., and Jani P.. 2005. Colonic carcinoma metastasis to the thyroid: a case of skip metastasis. J. Laryngol. Otol. 119:834–836. [DOI] [PubMed] [Google Scholar]
- 12. Werling, R. W. , Yaziji H., Bacchi C. E., and Gown A. M.. 2003. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 27:303–310. [DOI] [PubMed] [Google Scholar]
- 13. Nathanson, D. R. , A. T. 4th Culliford,, Shia J., Chen B., D'Alessio M., Zeng Z. S., et al. 2003. HER 2/neu expression and gene amplification in colon cancer. Int. J. Cancer 105:796–802. [DOI] [PubMed] [Google Scholar]
- 14. Carlsson, J. , Nordgren H., Sjostrom J., Wester K., Villman K., Bengtsson N. O., et al. 2004. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br. J. Cancer 90:2344–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]


