Abstract
Phenylpropanoids are a diverse chemical class with immense health benefits that are biosynthesized from the aromatic amino acid L-phenylalanine. This article reviews the progress for accessing variation in phenylpropanoids in germplasm collections, the genetic and molecular basis of phenylpropanoid biosynthesis, and the development of cultivars dense in seed-phenylpropanoids. Progress is also reviewed on high-throughput assays, factors that influence phenylpropanoids, the site of phenylpropanoids accumulation in seed, Genotype × Environment interactions, and on consumer attitudes for the acceptance of staple foods rich in phenylpropanoids. A paradigm shift was noted in barley, maize, rice, sorghum, soybean, and wheat, wherein cultivars rich in phenylpropanoids are grown in Europe and North and Central America. Studies have highlighted some biological constraints that need to be addressed for development of high-yielding cultivars that are rich in phenylpropanoids. Genomics-assisted breeding is expected to facilitate rapid introgression into improved genetic backgrounds by minimizing linkage drag. More research is needed to systematically characterize germplasm pools for assessing variation to support crop genetic enhancement, and assess consumer attitudes to foods rich in phenylpropanoids.
Keywords: anthocyanins, cereals, flavonoids, Genotype × Environment interaction, genetics and biosynthesis, germplasm, legumes, phenolics
Introduction
Polyphenols are secondary metabolites that are synthesized by plants from the amino acid phenylalanine. They are derived from the C6-C3 (phenyl-propane) skeleton. Plant biosynthesis produces various phenols that can be grouped generally as flavonoids and phenolics. Flavones, flavonols, flavanones, flavan-3-ols, anthocyanidins, isoflavones, coumarins, stilbenes, and lignans are the main flavonoids (Pereira et al., 2009). These are structurally distinct because of their specific hydroxylation, methylation, and conjugation patterns, with various monosaccharides and disaccharides (Graf et al., 2005; He and Giusti, 2010; Ignat et al., 2011; de Oliveira et al., 2014). Some flavonoids are exclusively synthesized by specific plants, such as phlobaphenes in maize (Sharma et al., 2012) and isoflavonoids in legumes (Mazur et al., 1998). Phenolic acids exist primarily as benzoic-acid and cinnamic-acid derivatives, and can occur in the free or conjugated forms. Gallic, p-benzoic, protocatechuic, syringic, and vanillic acids are benzoic-acid derivatives, while caffeic, ferulic, p-coumaric, and sinapic acids are cinnamic-acid derivatives (Razzaghi-As et al., 2013; de Oliveira et al., 2014).
Polyphenols are involved in various plant functions. They provide shades of color to flowers (color attracts pollinators), fruit, vegetables, and grains. Their bitter or astringent taste attributes can repel birds and other animals. Polyphenols protect plants from UV radiation and provide defense against environmental stress, as well as against pathogens and pests, and they act as signaling molecules to facilitate symbiotic nitrogen fixation and confer seed dormancy (Subramanian et al., 2007; Yang et al., 2008; Gu et al., 2011; Soares et al., 2013; de Oliveira et al., 2014). The phenylpropanoids have received considerable attention owing to their potential benefits for human health.
This article reviews the phenylpropanoid constituents, with emphasis on the mining of germplasm variation for flavonoids (excluding lignans and stilbenes) and phenolics, the site and factors that influence phenylpropanoid accumulation in seed, the Environment, Genotype, and Genotype × Environment interactions, the genetic and molecular basis of phenylpropanoid biosynthesis, and the development of seed-phenylpropanoid dense cultivars. Updates on flavonoids as promoters of micronutrient bioavailability and human health, high-throughput assays for estimation of phenylpropanoids, and consumer attitudes to accepting phenylpropanoid-dense foods are also included here.
Human health and micronutrient bioavailability
As a dietary component, phenylpropanoids have health-promoting properties due to their high antioxidant capacity that has been shown in both in-vivo and in-vitro systems (Cook and Samman, 1996). The antioxidant capacity of flavonoids is conferred by the high number of hydroxyl substitutions in each flavonoid molecule, which has a direct effect on the donating ability of hydrogen atoms to scavenge free radicals (Pietta, 2000). The food matrix and its processing conditions have strong effects on the retention of these compounds and their use as a functional food ingredient (Chavez-Santoscoy et al., 2016). Unfortunately, the availability of micronutrients and phytochemicals has been studied through reductionist and pharmacological approaches to date, while the beneficial effects of health-promoting compounds should in addition be analyzed holistically as food is not a drug. Furthermore, a compound that has been shown to be bioactive might have different effects when tested in a complex matrix (Fardet and Rock, 2014). Hence, it is important to identify and validate the stability of bioactive compounds both in isolation as well as once they have been incorporated into functional foods.
Combining cereals and legumes improves not only the nutritional content of these foods, but also their health-promoting effects. Anton et al. (2008) investigated the effects of red, black, and pinto or navy common bean (Phaseolus vulgaris L.) flour in wheat tortilla, and noted that tortillas with black beans had higher levels of crude protein, total phenols, and in-vitro antioxidant activity than those solely made with wheat flour. Black bean flavonoids, such as quercetin-3-O-glucoside, can have strong effects on down-regulation of expression of lipogenic proteins (Chavez-Santoscoy et al., 2014; Ramírez-Jiménez et al., 2015). Anthocyanin consumption from the original food matrix or once extracted inhibits tumorigenesis of esophageal cancer and changes inflammatory markers in rats (Peiffer et al., 2016). For example, 3-O-glucosylated anthocyanins (i.e., delphinidin, petunidin, malvidin) in the seed-coat extract of black violet beans is associated with antioxidant and anti-inflammatory activities (Oomah et al., 2010; Mojica et al., 2015). Furthermore, black-seeded common bean cultivars are superior to other food crops as nutraceutical supplements (Chavez-Santoscoy et al., 2013; Guajardo-Flores et al., 2015; Rosales-Serna et al., 2015).
Non-communicable diseases such as cancer, diabetes, heart disease, and stroke are some of the major challenges to global health, and they are often associated with the negative effects of globalization, rapid urbanization, diet, and increasingly sedentary lifestyles (Wagner and Brath, 2012). Flavonoids are implicated in the prevention of cardiovascular diseases (Mink et al., 2007; Curtis et al., 2009; Weseler et al., 2011). Soluble vascular adhesion molecule-1 (sVCAM-1) is an important biomarker that is used to predict the risk of death from coronary heart diseases (Blankenberg et al., 2001). Phenolic metabolites have stronger effects on reducing the sVCAM-1 levels than the corresponding flavonoids (Warner et al., 2016). Thus, the metabolism of flavonoids is critical to increases in their vascular efficacy, and this explains the differences among individuals when phenolic compounds are tested in-vivo. Colon cancer is a major public health burden in both developed and developing countries (Torre et al., 2015). Adoption of a Western diet is the major cause of colon cancer (Center et al., 2009). Sorghum [Sorghum bicolor (L.) Conrad Moench] flavonoids contribute to colon cancer prevention at concentrations that are achievable through the diet (Yang et al., 2014). They also noted that the composition of phenolic compounds, not content, has a major effect on estrogenic activity and on the protective efficacy of sorghum in preventing colon cancer.
Another global challenge to human health is being overweight or obese (Ng et al., 2014). Increased consumption of flavonoid-rich fruit and vegetables can help with weight management. Higher intake of foods rich in flavonols, flavan-3-ols, anthocyanins, and flavonoid polymers has been associated with less weight gain among men and women aged 27–65 years who were followed for up to 24 years (Bertoia et al., 2015). This association remained statistically significant for anthocyanins after further adjustment for fiber intake, which indicated that food sources with a high contents of anthocyanin and flavonoid polymers can be associated with less weight gain through mechanisms other than fiber content.
Phytic acid is the major contributor to reduced bioavailability of micronutrients in cereals and legumes, while polyphenols are major inhibitors of iron (Fe) absorption and act in a manner similar to phytate, by complexing Fe (Dwivedi et al., 2012). The metal-chelating characteristics of flavonoids are an important factor in antioxidant activities (Bonina et al., 1996; Boyle et al., 2000). Studies have demonstrated high binding capacities of polyphenols for Fe (Teucher et al., 2004; Perron and Brumaghim, 2009; Cercamondi et al., 2014). Flavonoids can bind nonheme iron and inhibit intestinal absorption of Fe from food (Mladenka et al., 2011; Corcoran et al., 2012). Nonheme iron is the principal form of Fe in plant foods, dairy products, and iron supplements. Flavonoids in colored bean seed coats strongly inhibit Fe bioavailability in bean digests (Hu et al., 2006). The chelation of metal ions by flavonoids can render the ions inactive in the generation of radicals, or alternately, flavonoids can themselves intercept radicals that are generated (Tako et al., 2015). Some polyphenols can reduce Fe(III) to Fe(II) (Perron and Brumaghim, 2009), thus promoting iron bioavailability. Catechin, 3,4-dihydroxybenzoic acid, kaempferol, and kaempferol 3-glucoside have been shown to promote Fe uptake, while myricetin, myricetin 3-glucoside, quercetin, and quercetin 3-glucoside inhibit Fe uptake (Hart et al., 2015). These inhibitors are, however, found in greater amounts than the promoters, which is consistent with the net inhibitory effects observed for black bean seed coats. The promotion of Fe uptake by some polyphenols and the identification of specific polyphenols that inhibit Fe uptake suggest the potential for breeding beans with improved Fe nutritional quality (Grieger et al., 2008; Tako and Glahn, 2010; Tako et al., 2015).
Flavonoids enhance the function of vitamin C, thus improving its absorption and protecting it from oxidation (Pietta, 2000). Vitamin C is a multi-functional micronutrient that is required in its reduced form (L-ascorbic acid) for many enzymatic reactions, and as a scavenger of free radicals generated from numerous physiological and biochemical processes (Evans and Halliwell, 2001). Flavonoids might also regenerate other antioxidants, such as tocopherols, by donating a hydrogen atom to the tocopheroxyl radical in a way that is reminiscent of their action on vitamin C (Boyle et al., 2000).
Analytical determination of phenylpropanoids
Various assays for determining phenolic compounds have been developed (Bravo, 1998; Khoddami et al., 2013). These can be classified as those that quantify the total phenolics content, or those that quantify or identify a specific group or class of phenolics. Colorimetric methods are used to determine the total phenolics levels, and high performance liquid chromatography (HPLC) is used to identify and quantify specific phenolic compounds. The colorimetric methods include Folin-Ciocalteu assays (Singleton et al., 1999), Prussian blue tests (Graham, 1992), ferric ammonium citrate tests (International Organization for Standardization), and vanillin-HCl and butanol-HCl tests (Price et al., 1978; Watterson and Butler, 1983; Porter et al., 1986). These methods have been used for total phenol determination in barley (Hordeum vulgare L.), common bean (Hart et al., 2015), rice (Oryza sativa L.; Begum et al., 2015), sorghum (Beta et al., 1999; Waniska and Rooney, 2000; Dykes et al., 2005; Dlamini et al., 2007; Chiremba et al., 2012), soybean [Glycine max (L.) Merr.; Nikolova et al., 2014; Phommalath et al., 2014], einkorn wheat (Triticum monococcum L.), and bread wheat (Triticum aestivum L.; Fogarasi et al., 2015). HPLC techniques coupled with photodiode array, fluorescence, or mass spectroscopy detectors have been used to identify and quantify specific phenolics in rice (Zhou et al., 2004), sorghum (Svensson et al., 2010; Chiremba et al., 2012), soybean (Kim et al., 2014; Kumar et al., 2015), and wheat (Ficco et al., 2014). Sriseadka et al. (2012) identified and quantified 11 flavonoids in black rice using liquid chromatography electrospray ionization tandem mass spectrometry, six of which were reported for the first time. Due to their chemical nature, the extraction method used, the standards used, and the presence of interfering substances, the various methods available for the analysis of phenylpropanoids remain too complex, time consuming, and labor intensive for routine screening. These limitations represent a bottleneck for modern studies of functional genomics and modern plant breeding (Furbank and Tester, 2011).
Near infrared (NIR) spectroscopy appears to be an appropriate technique to achieve these goals. It is faster than chromatographic or wet-chemical methods, and it can provide correct identification in less than 2 min without destroying the sample. NIR spectroscopy simultaneously measures several quality traits that are routinely tested in cereals (Bao et al., 2001, 2007; Wu et al., 2002; Wu and Shi, 2004, 2007; Osborne, 2006). It has been applied for the determination of phenolic compounds, flavonoid content, and antioxidant capacity in food derived from rice, sorghum, cocoa (Theobroma cacao L.), wine, grapes (Vitis vinifera L.), apples (Malus domestica Borkh., 1803), and tea [Camellia sinensis (L.) Kuntze; Whitacre et al., 2003; Cozzolino et al., 2004, 2008; Janik et al., 2007; Chen et al., 2008; Zhang et al., 2008; Pissard et al., 2013; Dykes et al., 2014; Hassan et al., 2015]. NIR spectroscopy has a high degree of precision when applied to the analysis of nutraceutical and antioxidant compounds in terms of their concentrations and antioxidant activities in foods (Ignat et al., 2011; Bunaciu et al., 2012; Lu and Rasco, 2012; Bittner et al., 2013; Cozzolino, 2015). Hence, NIR spectroscopy can provide very useful qualitative and quantitative information on different antioxidants, combined with its simplicity and low cost. Calibration development is critical to establishing a successful method based on NIR spectroscopy. Although the polyphenol quantification method is well established, a modified Folin-Cioalteu method incorporates the convenience of spectrometric measurements using 96-well microplates (Zhang et al., 2006). Automation of the workflow using 384-well microplates, as optimized for robotics, automated readers, and liquid handling systems, makes it possible to use much smaller quantities of reagents and solvents, and to significantly increase the throughput of the analysis of the compounds tested.
A 96-well microtiter assay that has been used for decades in the pharmaceutical industry has been standardized for screening total phenolic, flavonoid, and tannin contents, and for 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH)-scavenging activity in grape, sorghum (Herald et al., 2012, 2014; Bobo-García et al., 2015) and wheat (Cheng et al., 2006) extracts. This assay is thus as robust and reproducible as the conventional method for determining phenolic compounds. Most commonly used assays for measuring antioxidant activity, including those with DPPH, have both conceptual and technical limitations (Apak et al., 2013; Tian and Schaich, 2013; Xie and Schaich, 2014; Schaich et al., 2015), especially when comparing different food matrices. Although, it is necessary to continue investigating antioxidant efficacy using fundamental chemistry (Schaich et al., 2015), high-throughput assays remain a strategic asset to monitor variations in antioxidant activity in large germplasm collections. Laus et al. (2015) proposed the QUENCHERABTS for determination of antioxidant capacity, which is quick, easy, new, cheap, and reproducible. This method can also be used to accurately discriminate antioxidant capacity associated with the insoluble-bound phenolics of wheat grain without any preliminary sample extraction. Thus, it allows good discrimination among wheat genotypes, with better physiological significance than the classical Trolox equivalent antioxidant capacity and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) measurements. In addition, high-throughput oxygen-radical absorbance capacity assays conducted with more detailed and revised protocols might be a valuable alternative to the common testing methods for antioxidants (Huang et al., 2002).
Imaging systems working in the UV, visible, NIR, and Raman spectral ranges of the electromagnetic spectrum can be used to obtain information on composition and distribution of phenylpropanoids. As the hyperspectral imaging techniques combine spectroscopic and imaging systems, they can be used for detecting very low levels of chemical constituents in cereal and legume grain along with spatial distributions (Budevska, 2002; Kezhu et al., 2014; Mahajan et al., 2015). The hyperspectral microspectroscopic imaging techniques have been used to study the endosperm/aleurone/pericarp area of mature kernels of maize (Zea mays L.; Budevska, 2002). Hyperspectral imaging systems have also been used to develop single kernel methods to determine the physical and biochemical traits of cereals (Codgill et al., 2002, 2004; Fox and Manley, 2014). These methods focus on the calibration of the hyperspectral imaging instrument to predict the constituent concentrations in single kernels using NIR hyperspectral images.
Environment and genotype effects on phenylpropanoids
The flavonoid content is dominantly influenced by both genotype and environment. Better understanding of genotype and environment effects is a prerequisite to selecting food crops with enhanced flavonoids, so that cultivars high in flavonoids can be targeted to suitable environments.
Most studies on legumes as a source of functional foods has focused on soybean. For example, field research on six non-transgenic soybean genotypes grown in 23 environments (E) was carried out in Argentina to study seed nutraceutical composition. This showed that although Environment was the most important source, Genotype and Genotype × Environment interactions also had significant effects on grain nutraceutical composition (Carrera et al., 2014). These results agreed with those reported earlier by Lee et al. (2003), who showed that in South Korea, the main effects of Year, Site, Genotype, and all possible interactions between these were significant for all of the isoflavones. Murphy et al. (2009a) reported that breeding for relative isoflavone content was possible in two soybean populations in Ontario, Canada. Nonetheless, they cautioned that breeding for absolute stability is a challenge, because of the very strong effects of the environment on isoflavone accumulation in soybean. Temperature, precipitation, and soil moisture in the field conditions are the most important factors that influence flavonoid contents in soybean genotypes (Kim et al., 2012a), while temperature and soil moisture status change the isoflavone and anthocyanin contents of soybean under controlled conditions (Caldwell et al., 2005; Lozovaya et al., 2005; Chennupati et al., 2011).
Variable effects of genotype and environment on phenylpropanoid compounds were reported in wheat. Mpofu et al. (2006) and Fernandez-Orozco et al. (2010) showed greater contribution of environment than genotypes on flavonoid and phenolic content, while others reported greater genotypic effects than environment on polyphenols (Martini et al., 2015; Rascio et al., 2015). Variation in sowing date is also reported to cause significant differences in polyphenol content; i.e., polyphenols were increased in spring-sown compared to winter-sown wheats (Rascio et al., 2015). This variation depended on genotypes. A negative effect of spring sowing on grain yield was observed, but positive effects on 1000-kernel weight suggested that high temperatures can lead to a net accumulation of healthy substances in grain, but not a relative increase due to grain shriveling. There is a need to confirm these data in multiple environments, because such an approach facilitates the specific enrichment of cereal-based foods as per consumer requirements (Rascio et al., 2015). Total phenolics and phenolic acids were mostly affected by the environment in a 3-year field evaluation of durum wheat in Italy (Martini et al., 2015).
Changes in anthocyanin content of wheat cultivars were associated with sink-source (i.e., availability of carbohydrate for anthocyanin production) transition, grain position (i.e., the anthocyanin content decreased when grain position was more distal), and physiological stage of the crop. Magnesium fertilization and early harvest (at physiological maturity) increased anthocyanin content and concentrations by 65 and 39%, respectively (Bustos et al., 2012). Heat stress can adversely affect compounds that are beneficial or detrimental to human health (Dias and Lidon, 2010; Laino et al., 2010). More recently, de Leonardis et al. (2015) reported that in addition to affecting seed nutritional composition, 5-day heat stress (37°C) after flowering impacted on the antioxidant capacities and metabolic profiles of durum wheats. This response to heat stress was genotype-dependent, with most analyzed metabolites increasing in “Primadur” (high in seed carotenoids), but decreasing in “T1303” (high in seed anthocyanin).
Goufo and Trindade (2014) indicated that among four types of rice that were ranked by color, black rice cultivars were the highest in flavonoids, followed by the purple, red, and brown cultivars. These results were influenced by both the genotype and the environment. For example, elevated carbon dioxide reduced total phenolics, total flavonoids, and individual flavonoids (flavone, and some unidentified flavonoids) in rice kernels and all of the rice milling fractions. These results emphasize the importance of future atmospheric scenarios in breeding rice cultivars with increased antioxidant content (Goufo and Trindade, 2014; Goufo et al., 2014). The distribution of flavonoids in rice cultivars was not significantly affected by agronomic practices, but flavonoid content was significantly affected by the season, and the genotype, and by their interactions (de Mira et al., 2009; Liu et al., 2013).
Taleon et al. (2012) investigated the effects of both the genotype and environment on flavonoid concentrations in black sorghum grain in Texas. Significant variation due to the genotype, the environment, and their interactions was observed. Most of the variation was, however, associated with Genotype or Environment. The genotypic variation was greater than that for environment variation for flavones, while for flavanones, the environment variation was greater. Hence, the identification of both the best genotype and environment will provide the highest yields of total flavonoid content, and sorghum breeders need to evaluate these traits in multiple environments to select the genotypes with stable and high content of flavonoids of interest (Taleon et al., 2012). Similar results were reported and conclusions drawn for flavonoid content in red and lemon-yellow sorghum grain. The evaluation of genotypes in multiple environments was emphasized to obtain the best data related to the flavonoid content (Taleon et al., 2014).
Functional components including starch, protein, dietary fiber, and phenolic antioxidants were considerably influenced by the environment, genotype, type (i.e., hull-less, with hulls) and their interactions for barley grown in 23 different environments in eastern Canada (Abdel-Aal and Choo, 2014). The starch, which is the main available carbohydrate in barley, varied according to year, barley type and individual cultivars or lines. The absence of hulls tended to enhance the protein and total antioxidant capacity (Abdel-Aal and Choo, 2014).
Clearly, Environment, Genotype, and Genotype × Environment interactions have significant impact on phenylpropanoid constituents, which emphasizes the need for multi-environment testing to identify seed with phenylpropanoid-dense germplasm for use in plant breeding. Multi-environment testing across diverse agro-ecologies will also reveal which of the environments are more favorable for the production of phenylpropanoid-rich staple grain crops.
Factors influencing accumulation of phenylpropanoids
Crops can produce a large number of phenolic secondary metabolites that are not essential in the primary process of growth and development, but are of vital significance for their interactions with the environment and for their defense mechanisms (Cheynier et al., 2013). Flavonoids have relevant roles during the establishment of plants in the growing environment (Agati et al., 2013). The production of flavonoids is a response to developmental signals during seed development and to environmental signals, for protection. Thus, flavonoids are involved in protecting crops against major biotic and abiotic stresses (Liu et al., 2013). For some specific flavonoids, there is good understanding of the signals and activation of the phenolic biosynthetic genes (Cheynier et al., 2013). There are various biotic and abiotic stresses that influence the accumulation of specific flavonoids in crops (Supplementary Table 1).
Abiotic factors
Legumes
Drought stress decreased polyphenols in common bean seeds (Ovando-Martínez et al., 2014). Carbon dioxide and water stress increased the isoflavone content of soybean seed, but elevated temperature decreased total isoflavone content by about 65% (Caldwell et al., 2005). Water stress, elevated temperature, and solar radiation led to significant reductions in the specific and total content of isoflavones in soybean (Carrera and Dardanelli, 2015). Likewise, soil drought reduced the total phenolic content in the seeds of pea (Pisum sativum L.) and yellow lupin (Lupinus luteus L.; Juzoń et al., 2013). In mung bean [Vigna radiata (L.) R. Wilczek], water deficit reduced the total phenolics content (Afzal et al., 2014), while elevated UV-B radiation significantly reduced the concentrations of isoflavones and phenolic compounds in soybean seed (Kim et al., 2011). Genotypic differences in response to elevated ozone were noted across mung-bean cultivars, i.e., some cultivars were more sensitive to ozone (O3) stress (as measured by differences in antioxidants, metabolites, growth, total biomass, and yield) than others, suggesting the possibility of selection of suitable O3 resistant cultivars with improved phenylpropanoids in seeds for areas experiencing high concentation of O3 (Chaudhary and Agrawal, 2015).
Cereals
In maize, grain flavonoid increased considerably due to water stress, but the accumulation of phenolic compounds and carotenoids decreased (Ali et al., 2010). A marked increase in the total phenolics accumulation was observed in response to drought and salinity, and to a combination of these factors in barley kernels (Ahmed et al., 2013a). Salt increased the nutraceutical quality of mature grains in rice, as measured by total phenolics content, and anthocyanins and proanthocyanins (Chunthaburee et al., 2015), whereas drought led to increased total phenolic acids and carotenoids in wheat grain (Chakraborty and Pradhan, 2012). Stress as a result of nitrogen fertilization increased total free phenolic acids, but decreased conjugated soluble phenolic acids in wheat grain (Stumpf et al., 2015). Rice exposure to high CO2 resulted in decreased seed total phenolics content, with the highest reduction in sinapic and p-hydroxybenzoic acids. The total flavonoids content also decreased, with apigenin highly affected (Goufo et al., 2014). In whole rice kernels, γ-irradiation led to the accumulation of the main phenolic compounds (e.g., p-coumaric acid, ferulic acid), but it decreased anthocyanins (e.g., cyanidin-3-glucoside, peonidin-3-glucoside; Zhu et al., 2010). Wheat exposed to higher levels of solar UV radiation resulted in the production of red kernels and increased the concentrations of phenolic acids, flavonoids, and lutein (Lukow et al., 2012).
Biotic factors
Legumes
Seed flavonoids contribute to a constitutive defense mechanism, and they might accumulate after recurrent infection and as a result of several types of stress (Treutte, 2006). Seed concentrations of flavonoids, alkaloids, and terpenoids define the levels of effectiveness in the control of pathogens and insect pests in most legumes, and especially in common beans (Ndakidemi and Dakora, 2003). Seed accumulation of high amounts of phenolic compounds is toxic to bruchid [Callosobruchus maculatus (Fabricius 1775)] and provides resistance to storage pests in cowpea [Vigna unguiculata (L.) Walp], chickpea (Cicer arietinum L.), and soybean (Sharma and Thakur, 2014). Furthermore, flavonoids and isoflavonoids are considered to have major roles in host plant defense in the Fabaceae family (Mapope and Dakora, 2013). For example, the specific isoflavone content in legumes is strongly related to resistance to pathogens (Treutte, 2006). Rubiales et al. (2015) suggested a prominent role for flavonoid-related compounds in the specific defense against fungi, bacteria, and insects.
Cereals
Cell-wall phenolic acids in cereal grains are known to be associated with innate grain resistance to pests and pathogens (Santiago et al., 2013). For instance, the phenolic acids that are accumulated during wheat-kernel development contributed positively to Fusarium resistance (McKeehen et al., 1999). Analogous effects of Fusarium infection in barley showed that inoculation significantly reduced the ferulic acid content and increased the catechin content in the grain (Eggert et al., 2010). Increased accumulation of phenolic acids in maize pericarp is also associated with weevil [Sitophilus zeamais (Motschulsky, 1855)] resistance in tropical genotypes (García-Lara and Bergvinson, 2014). Similar findings in maize were reported for the effects of phenolic compounds against Angoumois grain moths [Sitotroga cerealella (Olivier, 1789); Ahmed et al., 2013b]. In contrast, phenolic acids, chlorogenic acids, and tannins were not involved in the infestation and damage caused by rice weevils [Sitophilus oryzae (Linnaeus, 1763); Bamisile et al., 2014].
Phenylpropanoid accumulation in seed
Cereal bran is rich in polyphenols, but these are usually removed from the grain before it is consumed as food. Most of the phytochemicals are lost following milling; thus, there is a trend to increase whole-grain consumption (Schaffer-Lequart et al., 2015). Wheat grain bran and germ contain up to 83% total phenolics, which is 15–18-fold higher on a μmol of gallic acid equiv 100 g−1 basis than in the endosperm fraction. Total phenolics progressively decreased during the progress in de-branning from the aleurone layer to the internal portions of the kernel (Adom et al., 2006).
The concentration, type and distribution of flavonoids differs among rice phenotypes. For example, proanthocyanidins are found in red kernels, anthocyanins in black grain, and phenolics in the non-pigmented counterparts (Abdel-Aal et al., 2006; Finocchiaro et al., 2007), whereas anthocyanins are found in the aleurone layer and the pericarp of purple, blue, and red wheat kernels (Havrlentová et al., 2014). Maize with red/ blue and blue kernels often contains a higher proportion of acylated anthocyanins than maize with red and purple kernels. Magenta-colored anthocyanins are concentrated in both the pericarp and aleurone layers, whereas blue maize grains accumulate pigments only in the aleurone layer (Žilić et al., 2012).
Matrix-assisted laser desorption/ionization coupled to imaging mass spectrometry allows simultaneous investigation of the content and spatial distribution of a wide range of biomolecules. Yoshimura et al. (2012) used this mass spectrometry technique to study the distribution of flavonoids in black-pigmented rice seeds, and they identified seven species of anthocyanin monoglycosides and two species of anthocyanin diglycosides. Anthocyanins composed of a pentose moiety (e.g., cyanidin-3-O-pentoside, petunidin-3-O-pentoside) were found throughout the pericarp, whereas anthocyanins composed of a hexose moiety (e.g., cyanidin-3-O-hexoside, peonidin-3-O-hexoside) were found only in the dorsal pericarp. Thus, anthocyanin species composed of different sugar moieties have different localization patterns in the pericarp of black rice. Galland et al. (2014) studied the localization, nature, and relative abundance of flavonoids in mature and germinated non-pigmented rice seeds of “Nipponbare” (a japonica cultivar) using a combination of confocal microscopy, mass spectrometry and gene expression analysis. They showed that matured rice seed exclusively accumulates flavones mostly in the embryo and to a lesser extent in the pericarp/testa. They detected 21 different flavones. Schaftoside and its two isomers were the major flavones in the embryo (54% of flavonoid compounds, as rhamnetin equivalents seed−1). Tricin and its conjugated derivatives accounted for 24% of the flavonoid signal distribution, making these the second largest contributor to the total flavone content of the embryo. In contrast, the pericarp/testa fraction accumulated exclusively schaftoside and two schaftoside isomers. The embryo has both O- and C-glycosylated flavones, while the pericarp/testa fraction accumulated only C-glycosylated flavones. The embryo flavone content is therefore very high when compared with that of the pericarp/testa in “Nipponbare” seeds.
Genetics and biosynthesis pathways
Genetics
Flavonoids in legumes
The concentration of isoflavones in soybean is a complex multi-genic trait. There are at least 50 quantitative trait loci (QTL) related to this trait (Meksem et al., 2001; Primomo et al., 2005; Gutierrez-Gonzalez et al., 2009, 2011; Zeng et al., 2009; Meng et al., 2011; Yang et al., 2011). Of these, two QTL with main effects that are located in Gm05 (LGA1) and GM08 (LGA2) consistently affected isoflavone content across environments (Gutierrez-Gonzalez et al., 2011). Isoflavone content in soybean is also affected significantly by additive genetic variance (Bi et al., 2015). Gutierrez-Gonzalez et al. (2010) found 35 main-effect genomic regions and many epistatic interactions that control genistein, daidzein, glycitein, and total isoflavone accumulation in soybean seeds. These findings suggest that a complex network of multiple minor-effect loci interconnected by epistatic interactions control isoflavone accumulation in soybean. The magnitude and significance of the effects of many of the nodes and connections in this network varied, however, according to the environment. This study made it possible to identify putative candidate genes for several main-effect and epistatic QTL and for known QTL (Gutierrez-Gonzalez et al., 2010). Wang et al. (2015) noted 34 QTL, of which 23 were new, for both individual and total seed isoflavone contents in soybean; while 6, 7, 10, and 11 QTL were associated with daidzein, glycitein, genistein and total isoflavone, respectively, in multi-generation soybean recombinant inbred lines (RILs; F5:6, F5:7, F5:8). Several DNA markers linked to QTL were identified across environments, thereby indicating that they can be used in the selection of segregants for higher isoflavone content, and also in map-based gene cloning.
Gutierrez-Gonzalez et al. (2010) showed that many enzymes in the phenylpropanoid pathway underlie QTL and modification of genes encoding for enzymes involved in this pathway might promote the biosynthesis of isoflavone in soybean seeds (Hao et al., 2008). Wang et al. (2014) found 33 expression QTL (eQTL) underlying the transcript abundance for the four gene families (PAL, CHS, IFS, F3H) on 15 chromosomes. Furthermore, the eQTL between Satt 278-Sat-134, Sat-134-Sct-010, and Satt 149-Sat-234 underlie the expression of both the IFS and CHS genes. More importantly, they identified five eQTL intervals that overlapped with phenotype QTL (pQTL), and a total of 11 candidate genes within the overlapped eQTL and pQTL.
Flavonoids in cereals
Polyphenol compounds that impart red pigment to wheat grain are synthesized through the flavonoid biosynthetic pathways. Himi and Noda (2005) showed that the expression of CHS, CHI, F3H, and DFR in the flavonoid pathway is completely suppressed indeveloping white grain, but not in red grain, in wheat. All four genes were highly up-regulated in the grain coat tissue of the red lines, whereas there was no significant expression in the white- colored lines, thus indicating that the R gene (Myb-type transcription factor) is involved in the activation of early flavonoid biosynthesis genes in wheat (Himi et al., 2005). Flavanone 3-hydroxylase (F3H) is a key enzyme at a divergence point of the flavonoid pathway that leads to the production of different pigments, proanthocyanidin, and anthocyanin. Himi et al. (2011) isolated F3H-A1, F3H-B1, and F3H-D1 on chromosomes 2A, 2B, and 2D of wheat. These genes were highly expressed in red grain and coleoptiles, and they appeared to be controlled by flavonoid regulators in each tissue. Moreover, the telomeric regions of the long arms of the chromosomes of homoeologous group 2 of wheat showed a syntenic relationship to the telomeric region of the long arm of rice chromosome 4, where the rice F3H gene is located. To date, a number of structural [Pal, Chs, Chi, F3h, F3′5′h, Dfr (TaDfr), Ans, Mt (Fmt), and Rt (3Rt)] and regulatory [Myc (TaMyc), Myb10 (Tamyb 10), and Mpc1] genes are known to be involved in flavonoid biosynthesis in wheat. In most cases, the information on the number of loci involved, chromosomal/intra-chromosomal localization, and sequences (complete or partial) of the gene copies are known (Khlestkina et al., 2015).
Jin et al. (2009) reported two QTL on chromosome 2, as qPH-2 for phenolic and qFL-2-1 for flavonoid content, which are flanked by CT87 and G1234, and which show large additive effects that account for 17 and 13%, respectively, of the phenotypic variation in rice. High narrow-sense heritability was estimated using 84 hybrids from an 11-parent diallel mating design, thus showing the importance of additive genetic variance for total phenols in maize (Mahan et al., 2013). A genome-wide association study that involved a global sorghum diversity panel (n = 381) and 404,628 SNP markers (Rhodes et al., 2014) identified novel QTL associated with polyphenols in sorghum. Some of these were co-localized with homolog of flavonoid pathway genes from other plants, including an ortholog of maize Pr1 and a homolog of Arabidopsis TT16. General linear models (GLMs) did not precisely map a loss-of-function allele of the Tannin 1 gene (tan 1), while either a GLM accounting for population structure or a standard linear model considering kinship did identify it (Morris et al., 2013). Furthermore, tan 1 was accurately mapped using a simple loss-of-function genome scan for the genotype-phenotype co-variation only in the putative loss-of-function allele.
Anthocyanins in cereals
The deposition of proanthocyanidins in the seed testa results in red grain whereas anthocyanins in the pericarp and aleurone layer give rise to purple and blue colored wheat kernels, respectively (Zeven, 1991). Ba1 (Keppenne and Baenziger, 1990) and Ba2 (Dubcovsky et al., 1996) were found to control blue grain in a tall wheat grass [Thinopyrum ponticum (hereonward referred as Th. ponticum) = Agropyron elongatum] and in Triticum monococcum, respectively. These two genes were physically mapped: Ba1 at FL0.71-0.80 on chromosome 4Ag (Zheng et al., 2006), and Ba2 near the centromere on chromosome 4AL (Dubcovsky et al., 1996). The wild relative Th. bessarabicum bears the gene BaThb, which produced blue grain and has been physically mapped between the centromere and FL0.52 on chromosome arm 4JL. BaThb differs from Ba1 and Ba2, and has a strong dose effect, thus confirming Th. bessarabicum as another source of blue aleurone grain in wheat (Shen et al., 2013). To date, several blue wheat elite lines have been developed. These lines carry Th. ponticum or T. monococcum introgressed chromosomes. Burešováet et al. (2015) found that 17 of 26 such lines have introgression from Th. ponticum, while the remaining bear T. monococcum chromatin. This finding suggests that these blue aleurone wheat lines show major differences in chromatin composition. Introgression activates the blue aleurone trait, which is inactivated in bread wheat germplasm lacking the Th. ponticum chromosome segment.
The genes Pp1 and Pp3 mapped on the short chromosome arms of the homeologous group 7 and on chromosome arm 2AL, respectively, control purple grain color in wheat (Dobrovolskaya et al., 2006; Khlestkina et al., 2010; Tereshchenko et al., 2012). Furthermore, the Pp1 genes are orthologs to both maize C1 and rice OsC1, and encode MYB-like transcription factors that activate structural genes related to enzymes associated with anthocyanin biosynthesis (Khlestkina, 2013). Pp3 is orthologous to Ra in rice (Wang and Shu, 2007) and Lc in maize (Ludwig et al., 1989). It encodes TaMYC1, which is strongly expressed in the pericarp (Shoeva et al., 2014). Pp1 and Pp3 upregulate the transcript abundance of structural genes Chi (Chalcone-flavone isomerase) and F3h (flavanone 3-hydroxylase) in the pericarp of near isogenic lines carrying various combinations of Pp alleles (Gordeeva et al., 2015).
The pericarp of red rice grains accumulates proanthocyanidin (Sweeney et al., 2006), while purple rice grain accumulate anthocyanin (Rahman et al., 2013). Maeda et al. (2014) confirmed that Pp on chromosome 1 and Pb on chromosome 4 acted together to influence grain color (Wang and Shu, 2007; Rahman et al., 2013). They also indicated that Kala1, Kala3, and Kala4 are essential for black pigmentation. Their loci were mapped between RM7405 and RM7419 on chromosome 1, between RM15008 and RM 3400 on chromosome 3, and between RM1354 and RM7210 on chromosome 4, respectively. Ectopic expression of the Kala4 bHLH gene leads to expression of anthocyanin biosynthesis genes in the pericarp, and produces black rice grains, while a DNA duplication event at the 5′-end of the gene that correlated with kala4 expression also controls black grain (Oikawa et al., 2015).
Wei et al. (2013) showed that in barley a dominant gene Blp mapped on chromosome 1HL controls black grain, while the complementary dominant genes Pre1 and Pre2 mapped on chromosome 2HL determine the purple color. They also indicated that the complementary dominant genes Blx1, Blx3, and Blx4 mapped on chromosome 4H, plus Blx2 and Blx5 mapped on chromosome 7HL, are responsible for blue colored barley kernels.
Anthocyanin biosynthesis in maize is regulated by interactions between two sets of transcription factors that are encoded by c1/pl1 and r1/b1; c1 and r1 regulate pigmentation in the kernel aleurone, and pl1 and b1 regulate it in the plant body (Chandler et al., 1989). The pr1 gene has a role in the cl- and rl-regulated anthocyanin biosynthesis pathway (Sharma et al., 2011). The pr1 locus accumulates red (pelargonidin) and the Pr1 accumulates purple (cyanidin) anthocyanins in the aleurone cells of seeds. The putative F3′H encoding gene (Zmf3′h1) was mapped on chromosome 5L, while purple and red anthocyanins accumulated in Pr1 and pr1 lines, respectively. Furthermore, pr1 has four alleles, which are characterized by insertion or deletion polymorphisms that co-segregated with the red aleurone phenotype in the F2 population containing Pr1 and pr1 alleles. This gene is under the regulatory control of anthocyanin transcription factors red1 and colorless1. Moreover, Sharma et al. (2012) showed that Zmf3′h1 also participates in biosynthesis of phlobaphenes and 3-deoxyflavonoid compounds, which accumulate in maize pericarp and cob glumes and silks, and are under regulatory control by P1. Thus, Zmf3′h1 has a significant role in generation of diversity for anthocyanin, phlobaphenes, 3-deoxyanthocyanidin and C-glycosyl flavone compounds; the latter two of these compounds impart maize plant resistant to pests and pathogens (Nicholson and Hammerschmidt, 1992; Byrne et al., 1996).
PERICARP COLOR 1 (P1) is an R2R3-MYB type transcription factor that controls the accumulation of brick red phlobaphenes pigments in grain pericarp in maize. Phlobaphenes are polymers of the flavan-4-ols apiforol and luteoforol, and are generated from naringenin or eriodictyol by dehydroflavonol reductase (DFR), which is encoded by maize A1. P1 alleles specify different pericarp and cob glume colors. For example, P1-ww results in white pericarps and white cob glumes, whereas P1-rr produces red pericarps and red cob glumes. A1 mutants in a P1-rr background (P1-rr; a1) display an unidentified brown pigment that contrasts with the white P1-ww pericarp, thereby suggesting metabolic shunting toward a different branch of the flavonoid pathway (Casas et al., 2014). Most of the elite lines used in the production of hybrid maize lack flavones. Casas et al. (2014) showed that maize lines harboring the P1-rr allele in combination with recessive a1 accumulate flavones to the same levels as flavone-rich vegetables. These results suggest that nutritionally beneficial flavones can be re-introduced into elite lines to increase the dietary benefits of maize.
Clearly, over the years a greater understanding of phenylpropanoid genetics has been achieved, and this knowledge-based inheritance can now facilitate the enriching of staple grain crops with health-promoting compounds.
Biosynthesis pathways
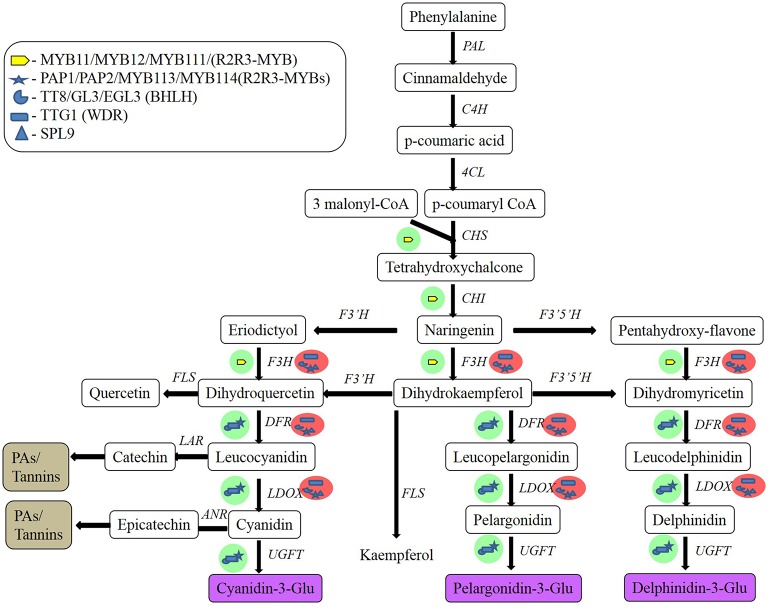
Flavonoid biosynthesis (Figure 1) begins with the phenylpropanoid pathway, in which phenylalanine is converted into p-coumaroyl CoA. This pathway is mediated by the flavonoid metabolon, which is attached to the cytoplasmic face of the endoplasmic reticulum. Metabolons are multienzyme complexes. They represent highly organized assemblies of sequential enzymes in a metabolic pathway, and they provide increased metabolic efficiency and higher substrate selectivity (Kaur-Sawhney et al., 2003). The basic carbon structure of flavonoids is generated by a two-step condensation process that is mediated by chalcone synthase (CHS) and chalcone isomerase (CHI). The resulting colorless naringenin is then oxidized by F3H to dihydrokaempferol. Naringenin can also be directly hydroxylated to yield dihydroflavonols, and then later converted into anthocyanidins. Despite the central biosynthetic pathway being conserved in plants, various enzymes can modify the basic flavonoid skeletal structure including reductases, isomerases, hydroxylases, and dioxygenases, to form different subclasses of flavonoids in different species. Transferases add groups like sugars, methyl, or acyl groups to the backbone structure. The synthesis of proanthocyanidins branches off from the anthocyanin pathway subsequent to the reduction of dihydroquercetin to leucocyanidin. The two major enzymes involved in the formation of proanthocyanidins are leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) (Bogs et al., 2005).
Figure 1.
Flavonoid biosynthetic pathway in plant cells and regulatory gene regulation of this pathway in Arabidopsis. Green and red circles indicate activation and repression, respectively. MYB, myeloblast; PAP, production of anthocyanin pigment; TT8, transparent testa A8; bHLHs, basic helix-loop-helix proteins; TTG1, transparent testa glabrous1; SPL9, squamosa promoter binding protein-like 9; PAL, phenyalanine ammonia-lyase; C4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumarate CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3′-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′5′-hydroxylase; DFR, dihydroflavonol reductase; LDOX, leucoanthocyanidin oxidase; UGFT, UDP-glucose flavonoid 3-O-glucosyl transferase; FLS, flavonol synthase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase (After Pandey et al., 2014).
The flavonoid biosynthesis pathway is extensively regulated by transcription factors, such as the MYB proteins, basic helix-loop-helix (bHLH) factors, and WD-repeat-containing proteins. Transcriptional regulation has been extensively investigated in maize and Arabidopsis. This has facilitated the identification of differences in regulation between monocots and dicots (Ferreyra et al., 2012). The MYB domain is made up of one (MYBR1), two (R2R3-MYB), or three (MYB3) repeats of about 52 amino acids, with R2R3-MYB being the most predominant. Specific motifs and conserved residues mean that these proteins can regulate single branches of the flavonoid pathway (Hichri et al., 2010; Lin-Wang et al., 2010). The overexpression of VlMYBA-1 in the hair roots of grapevine induces the expression of only the genes involved in anthocyanin biosynthesis and transport, whereas the overexpression of VvMYBPA1 and VvMYBPA2 selectively activates genes involved in the synthesis of proanthocyanidins. Albert (2015) isolated Tr-MYB133 and Tr-MYB134 in white clover (Trifolium repens L.), which encode R2R3-MYBs that antagonize the activity of MBW activation complexes. These two genes are also conserved in other legume species, and form two subclades within the larger anthocyanin/proanthocyanidin clade of MYB repressors. However, unlike petunia (Petunia sps.) and Arabidopsis, these R2RS-MYB repressors do not prevent ectopic accumulation of anthocyanins or proanthocyanidins. Instead, they are expressed when anthocyanins or proanthocyanidins are synthesized, and provide feedback regulation to MBW complexes. This feedback occurs because Tr-MYB133 and Tr-MYB134 are themselves regulated by MBW complexes. Tr-MYB133 is regulated by MBW complexes that contain anthocyanin-related R2R3-MYB proteins (Tr-RED LEAF), while Tr-MYB134 is regulated by complexes containing the proanthocyanidin R2RS-MYBs (Tr-MYB14). Thus, regulation of Tr-MYB133 and Tr-MYB134 by pathway-specific MBW complexes results in anthocyanin or proanthocyanidin synthesis (Albert, 2015).
The bHLH proteins are ubiquitous transcription factors that are found across eukaryotes, from yeast to human. They are widely distributed in plants and are characterized by the presence of a critical region, called the bHLH domain. The basic region of the bHLH domain consists of 15–17 amino acids, and this is responsible for DNA binding and activation. The bHLH proteins form heterodimers and function as repressors in the absence of the basic region (Toledo-Ortiz et al., 2003). The first 200 amino acids of the protein on the N'-terminal are referred to as MIR (MYB–interacting region), and the next 200 amino acids are known as the WD-40/AD domain, which facilitates the formation of ternary complexes known as MBW complexes in plant species. The bHLH proteins can bind DNA as a single molecule or as a dimer, with MYB proteins based on the promoter target (Hichri et al., 2011). Unlike MYB proteins, some bHLH proteins can influence more than one branch of the flavonoid pathway. The TT8 (transparent testa 8) factor of Arabidopsis is an example of the regulation of both the anthocyanin and proanthocyanidins pathways.
Ternary complexes that comprise the above-mentioned transcription factors that are referred to as MBW complexes have been comprehensively identified in model plants and crops (Supplementary Table 2). In Arabidopsis, the MYB protein controls the target-gene specificity of the ternary complex. The presence of PAP1/PAP2 (production of anthocyanin pigment), TT2 (transparent testa 2), GL1 (glabrous 1), WER (werewolf), and AtMYB61 regulate anthocyanin accumulation in seedlings, proanthocyanidins biosynthesis in seed integuments, trichome formation, root-hair initiation, and mucilage production in seed integuments, respectively (Baudry et al., 2004). Flavonoid biosynthesis is also regulated by environmental factors, such as light and external stresses in grape (Vitis vinifera L.; Li et al., 2014), and the intensity of light and sucrose conditions in Arabidopsis (Das et al., 2012), which is mediated by the MYBL2 factor. In mulberry (Morus spp.), transcriptional levels of regulatory genes involved in the biosynthesis of anthocyanins are directly related to the degree of ripening and the coloration intensity of the fruits (Qi et al., 2014).
In summary here, elucidating the intricate regulatory patterns of the flavonoid biosynthesis pathway will pave the way for genetic enhancement. Understanding how biosynthetic enzymes are regulated and their spatio-temporal organization will enable modification of the patterns of flavonoid expression and accumulation. Manipulation of the pathways can generate fruit and vegetables enriched in antioxidant and nutritional compounds, as well as provide other medicinal benefits. Future research should be aimed at delineation of the factors that control the expression of the regulatory genes, and also at understanding the allelic variability between cultivars of the same species to identify useful DNA markers as aids for indirect selection in breeding.
Germplasm mining for variations in phenylpropanoids
Flavonoids in legumes
A systematic search reveals that there have been limited germplasm accessions evaluated for flavonoids among grain legume crops (Table 1). Up to two-fold variations in flavonoid content were noted in cowpea, groundnut (Arachis hypogaea L.), guar [Cyamopsis tetragonoloba (L.) Taub.], mung bean, and soybean germplasm. The differences in chickpea and lima bean (Phaseolus lunatus L.) germplasm were 76- and 86-fold, respectively. In common bean, 2–13-fold differences were reported among landraces and wild and weedy types, with the latter showing maximum fold differences in flavonoid content. Kaempferol and quercetin were the main flavonoid compounds in common bean (Espinosa-Alonso et al., 2006; Mishra et al., 2012). Four-fold differences were found in cowpea and 6–76-fold in common bean germplasm. In cowpea, seed color and content of flavonol were correlated, with red-seeded accessions containing greater flavonol than white-seeded cowpeas. Other seed color types had limited variations, except for light-brown-seeded accession IAR 48, which showed exceptionally high flavonol content [0.796 mg g−1 dry weight (DW)] among light-brown-seeded cowpeas (Ojwang et al., 2012). Quercetin was the most abundant flavonol in cowpea (Ojwang et al., 2012), while kaempferol and quercetin flavonol were the most abundant flavonols in common bean (Doria et al., 2012).
Table 1.
Germplasm-wide variations in total phenylpropanoid constituents in food legumes.
| Germplasm (no.) | Variation for flavonoids | References |
|---|---|---|
| FLAVONOID | ||
| Common bean (Phaseolus vulgaris L.) | ||
| Landraces (20) | 0.05–0.41 mg quercetin (QUE) equivalent (QUAE) g−1 DW | Mishra et al., 2012 |
| Wild and weedy types (64) | 0.008–0.106 mg g−1 FW, G 12896-B and G 11025B being highest | Espinosa-Alonso et al., 2006 |
| Zolfino landraces (4) | 0.302–0.711 mg g−1 FW | Romani et al., 2004 |
| Chickpea (Cicer arietinum L.) | ||
| Landraces (20) | 0.05–0.41 mg quercetin equivalent (QUAE) g−1 DW | Mishra et al., 2012 |
| Cowpea (Vigna unguiculata (L.) Walp.) | ||
| Black, red, tan, and white grains (8) | 0.253–0.442 mg g−1 DW, quercetin being highest (0.214–0.279 mg) | Wang et al., 2008 |
| Groundnut (Arachis hypogaea L.) | ||
| Black, pink, red, tan, and white grains (8) | 0.138–0.336 μg g−1 DW, quercetin being highest (0.133–0.288 mg) | Wang et al., 2008 |
| Guar (Cyamopsis tetragonoloba (L.) Taub.) | ||
| Accession (36) | 13–23 mg 100 g−1 DW; Kaempferol, the major component (10.7–19.8 mg) | Wang and Morris, 2007 |
| Lima bean (Phaseolus lunatus L.) | ||
| Black, brown, pink, red, and white-grains (50) | 0.2–17.3 mg rutin equiv. (RUE) g−1 DW | Agostini-Costa et al., 2015 |
| Mung bean (Vigna radiata (L.) R. Wilczek) | ||
| Accession (50) | 1.204–2.932 mg g−1 DW | Kim et al., 2013 |
| Soybean (Glycine max (L.) Merr.) | ||
| Black, brown, green, red, and yellow grains (8) | 0.892–0.916 mg g−1 DW; genistein (0.438–0.458 mg) and daidzein (0.315–0.354 mg) greater than Kaempferol (0.038–0.068 mg) | Wang et al., 2008 |
| FLAVONOL | ||
| Common bean | ||
| Gene pools differing in seed color (16) | 0.002–0.125 mg g−1 DW | de Lima et al., 2014 |
| Landrace-based populations (10) | 0.011–0.081 mg g−1 DW | Doria et al., 2012 |
| Zolfino landraces (4) | 1.18–7.09 mg g−1 FW | Romani et al., 2004 |
| Cowpea (Vigna unguiculata (L.) Walp.) | ||
| Black, brown, green, golden, and white grains (10) | 0.27–1.06 mg g−1 DW, red-seeded had greater (mean 0.97 mg) than white-seeded (0.27 mg) | Ojwang et al., 2012 |
| ISOFLAVONE | ||
| Common bean | ||
| Gene pools differing in seed color (16) | 0.0008–0.14 mg g−1 DW | de Lima et al., 2014 |
| Landrace-based populations (10) | 0.009–0.113 mg g−1 DW | Doria et al., 2012 |
| Zolfino landraces (4) | 0.002–0.015 mg g−1 FW | Romani et al., 2004 |
| Soybean | ||
| Indian and exotic accessions (46) | 0.234–2.092 mg g−1 DW | Kumar et al., 2015 |
| 0 to VI maturity groups (40) | 0.551–7.584 mg g−1 DW | Zhang et al., 2014 |
| Cultivars (44) | 0.276–1.709 mg g−1 DW | Kim et al., 2014 |
| Seed size variations (204) | 0.682–4.778 mg g−1 DW | Kim et al., 2012b |
| 0 to II maturity groups (210) | 1.161–2.743 mg g−1 DW | Wang et al., 2000 |
Original data on phenylpropanoid constituents given in papers cited here were converted and presented into mg g-1 dry weight.
Multi-fold differences were noted in isoflavone content among common bean germplasm, with black-, ivory-, and brown-yellow-seeded accessions showing the highest isoflavones (Doria et al., 2012; de Lima et al., 2014). There were 2–14-fold differences in isoflavone content in soybean. Daizin, genistin, glycitin, malonyldaidzin, malonylglycitin, and malonylgenistin were the major moieties (Kim et al., 2012a; Zhang et al., 2014). Maturity groups (MG) had significant differences in isoflavone content in soybean. For example, MG V and VI had significantly higher total isoflavones compared to MG 0 to IV. Differences among MG 0 to IV or between MG V and VI were, however, not statistically significant (Zhang et al., 2014). Seed size influenced mean isoflavone content among geographically different soybean accessions. For example, small-seeded accessions from North America and Korea had similar isoflavone (2.53, 2.56 mg g−1, respectively), the medium-seeded accessions from North America and Korea had higher isoflavone (2.24, 2.48 mg g−1, respectively) than those from China (1.38 mg g−1), while large-seeded Korean accessions had higher isoflavone (1.83 mg g−1) levels than those from North America and China accessions (1.20, 1.34 mg g−1, respectively; Kim et al., 2012b).
Flavonoids in cereals
Barley, rice, sorghum, and wheat germplasm/cultivars have been studied for variations in flavonoids (Table 2). About 2-fold variation among barley and up to 21-fold variation among rice germplasm were noted. Black-grained and red-grained rice accessions had greater flavonoids than white-grained types (Shen et al., 2009; Shao et al., 2014a). Wheat germplasm that differed in grain color had relatively narrow genetic differences for flavonoids. Up to 12-fold difference in flavone and up to 6-fold difference in flavanone content were observed among sorghum open-pollinated and hybrid cultivars.
Table 2.
Germplasm-wide variations in total phenylpropanoid constituents in staple cereals.
| Germplasm (no.) | Variation for flavonoids | References |
|---|---|---|
| FLAVONOID | ||
| Barley (Hordeum vulgare L.) | ||
| Landraces (37) | 0.47–1.23 mg catechin equival. (CE) mg g−1 dry weight (DW) | Abidi et al., 2015 |
| Hulled and hull-less (11) | 27–66 mg quercetin equiv. (QUE) g−1 extract | Mahmoudi et al., 2015 |
| Rice (Oryza sativa L.) | ||
| Diverse accessions (20) | 0.19–3.28 and 0.20–3.54 mg CE g−1 DW in two seasons | Shao et al., 2014a |
| Cultivars with pigmented and non-pigmented grains (11) | 0.0012–0.0258 mg QE g−1 bran; higher flavonoid in pigmented than non-pigmented; greater flavanol in black-colored indica than black-colored japonica | Huang and Ng, 2012 |
| Black, red, and white grains (481) | 0.89–2.86 mg Rutin equiv. (RE) g−1 DW, average values greater in black (0.24 mg) than red (0.15 mg) and white (0.13 mg) grains | Shen et al., 2009 |
| Wheat (Triticum aestivum L.) | ||
| Black, purple, and white grains (4) | 0.236–0.319 mg RE g−1 DW, with black grains being highest in flavonoid | Li et al., 2015 |
| FLAVONE AND FLAVANONE | ||
| Sorghum (Sorghum bicolor (L.) Conrad Moench) | ||
| Colored grains (12) | Flavone: 0.008–0.1 mg g−1 DW; flavanone: 0.008–0.048 mg g−1 DW | Dykes et al., 2009 |
| Black grained lines and hybrids (8) | Flavone: 0.018–0.056 mg g−1 DW; flavanone: 0.089–0.119 mg g−1 DW | Dykes et al., 2013 |
Original data on phenylpropanoid constituents given in papers cited here were converted and presented into mg g-1 dry weight.
Anthocyanin in legumes
Nine-fold differences in anthocyanin levels were noted among common bean germplasm lines differing in seed color and weight. Accessions with brown, red, or black seed color had higher levels of anthocyanins (0.003–0.005 mg g−1 DW; Akond et al., 2011). Kidney-bean germplasm showed large range variations in total anthocyanins (0.07–2.78 mg g−1 DW), black-seeded types being richer sources of anthocyanins than red- or brown-seeded types (Choung et al., 2003). About 2-fold differences in total anthocyanin were recorded among cowpea lines. Black-seeded cultivars had higher levels of anthocyanins than the green-seeded type. The predominant anthocyanin compounds include delphidin-3-O-glucoside, cyanidin-O-glucoside, petunidin-O-glucoside, and malvidin-O-glucoside (Ojwang et al., 2012). In soybean, several-fold differences (30–213 times) were noted among Chinese and Japanese cultivars and landraces, with cyanidin-3-glucoside being the most abundant (Zhang et al., 2011; Phommalath et al., 2014).
Anthocyanins in cereals
Variations in anthocyanin levels in germplasm/cultivars have been reported for barley, maize, rice, sorghum, and wheat (Table 3). Two-fold to eighty-fold variation in anthocyanin concentration was noted among barley germplasm. Purple-grain and blue-grain barley groups had significantly greater mean anthocyanin levels (0.32 mg g−1) than black barley (0.04 mg g−1). The most common anthocyanins in purple barley were cyanidin-3-glucoside, peonidin-3-glucoside, and pelargonidin-3-glucoside, whereas delphinidin-3-glucoside was the most abundant anthocyanin in blue and black barley groups (Kim et al., 2007). The predominant anthocyanins were delphidin-3-malonylglucoside and cyanidin-3-malonylglucoside, followed by delphidin-3-glucoside and cyanidin-3-glucoside in blue barley (Diczházi and Kursinszki, 2014). In maize, multi-fold differences in total anthocyanins, with most reporting 15–36-fold were observed among colored-grain accessions. Cyanidin-glucoside was the major anthocyanin (Lopez-Martinez et al., 2009; Kuhnen et al., 2011; Mendoza-Díaz et al., 2012; Žilić et al., 2012). Maize with dark-red, blue, or purple grain colors holds immense promise for the development of functional foods and natural colorants. Several-fold differences, which ranged from 4 to 121 times, were noted among pigmented rice germplasm. Cyanidin-3-glucoside, peonidin-3-glucoside, cyanidin diglucoside, and malvidin were the major anthocyanins in black-rice and red-rice kernels (Ryu et al., 1998; Abdel-Aal et al., 2006; Lee, 2010; Zhang et al., 2010; Chen et al., 2012). Red-grained and black-grained sorghum cultivars showed up to 21-fold differences in anthocyanins.
Table 3.
Germplasm-wide variations in total phenylpropanoid constituents in staple cereals.
| Germplasm (no.) | Variation in total anthocyanin content | References |
|---|---|---|
| Barley (Hordeum vulgare L.) | ||
| Colored grains (4) | 0.047–0.084 mg g−1 dry weight (DW)$ | Diczházi and Kursinszki, 2014 |
| Hulled and unhulled colored grains (127) | 0.013–1.038 mg catechin equiv. g−1 DW | Kim et al., 2007 |
| Maize (Zea mays L.) | ||
| Blue-grain hybrids/varieties (7) | 0.65–1.05 mg cyaniding 3-glucoside (Cy3Glu) equiv. g−1 DW | Urias-Lugo et al., 2015 |
| Waxy maize (49) | 0.07–1.06 mg Cy3Glu equiv. g−1 DW | Harakotr et al., 2015 |
| Colored grains (4) | 1.74–9.63 mg Cy3Glu equiv. g−1 DW | Mendoza-Díaz et al., 2012 |
| Colored grains (10) | 0.002–0.696 mg Cy3Glu equiv. g−1 DW | Žilić et al., 2012 |
| Red and blue grains (9) | 0.02–0.72 mg Cy3Glu equiv. g−1 DW | Montilla et al., 2011 |
| Waxy colored and normal yellow grains (3) | 0.001–2.761 mg Cy3Glu equiv. g−1 DW | Hu and Xu, 2011 |
| Colored grains (18) | 0.30–8.50 mg Cy3Glu equiv. g−1; purple, 0.93 to8.50 mg; black, 0.76-1.20 mg; Red, 0.85–1.54 mg | Lopez-Martinez et al., 2009 |
| Colored grains (9) | 0.051–1.277 mg g−1 DW$ | Abdel-Aal et al., 2006 |
| Rice (Oryza sativa L.) | ||
| Colored grains (9) | 0.21–2.98 mg g−1 DW$ | Chen et al., 2012 |
| Black and red grains (13) | Black grains: 1.09–2.56 mg Cy3Glu equiv. 100 g−1 DW; Red grains: 0.003–0.014 mg Cy3Glu equiv. g−1 DW | Sompong et al., 2011 |
| Black grains (12) | 12.31–51.01 mg Cy3Glu equiv. g−1 DW | Zhang et al., 2010 |
| Black grains (10) | 0.052–1.684 mg Cy3Glu equiv. g−1 DW | Lee, 2010 |
| Wild rice with colored grains (3) | 0.027–3.276 mg g−1 DW$ | Abdel-Aal et al., 2006 |
| Sorghum (Sorghum bicolor (L.) Conrad Moench) | ||
| Colored grains (12) | 0.032–0.68 mg g−1 DW$ | Dykes et al., 2009 |
| Black grained lines and hybrids (8) | 0.33–1.05 mg g−1 DW$ | Dykes et al., 2013 |
| Wheat (Triticum species) | ||
| Durum and bread wheat colored grains (76) | Blue colored bread wheat, 0.082–0.174 mg g−1 DW, mean 0.118 mg; purple and red colored durum wheat, 0.008–0.05 (mean, 0.023), and 0.001–0.025 mg (mean, 0.01), respectively$ | Ficco et al., 2014 |
| Colored grains (4) | 0.007–0.12 mg g−1 DW$ | Žofajova et al., 2012 |
| Pigmented grains (13) | 0.0034–0.0752 cyanidin glucoside equiv. mg g−1 DW | Eticha et al., 2011 |
| Colored grains (7) | 0.007–0.212 mg g−1 DW$ | Abdel-Aal et al., 2006 |
Original data on phenylpropanoid constituents given in papers cited here were converted and presented into mg g-1 dry weight.
total anthocyanin (not the anthocyanin compounds) value was given in the original literature.
Wheat germplasm lines and cultivars showed up to 30-fold difference in anthocyanin content. Blue and purple grain accessions had higher levels of anthocyanins (Abdel-Aal et al., 2006; Eticha et al., 2011; Žofajova et al., 2012; Ficco et al., 2014). Five to eight anthocyanin compounds were noticed in blue grain wheat extracts, compared to three anthocyanin compounds in purple and red wheat (Ficco et al., 2014). Delphinidin-3-O-rutinoside, delphinidin 3-O-glucoside, and malvidin-3-O-glucoside were the predominant anthocyanins in blue wheat, while cyanidin-3-O-glucoside, peonidin-3-O-glucoside, and malvidin-3-O-glucoside were found in purple wheat (Abdel-Aal et al., 2006; Ficco et al., 2014). Zeven (1991) gave details on the origin and history of wheat with blue and purple grains.
Phenolics in legumes
Table 4 gives the variations reported for phenolics in grain legumes. Among lima bean germplasm, there was 2–4-fold variation for phenols, except for one study that indicated a very high level (97-fold; Agostini-Costa et al., 2015). Ferulic acid was the most abundant, followed by p-coumaric and sinapic acids (Espinosa-Alonso et al., 2006; Luthria and Pastor-Corrales, 2006). Black, brown and red common beans had higher phenols than white grain types (Akond et al., 2011; Agostini-Costa et al., 2015). Up to 13-fold differences in total phenols were noted among chickpea germplasm with colored grains. Desi types had higher levels of phenols compared to Kabuli types. Among the anatomical parts, the seed coat was the major source of variation for total phenols. Cowpea germplasm and cultivars showed 3–11-fold differences in total phenols. Protocatechuic acid was the major phenolic, while p-hydroxybenzoic, caffeic, p-coumaric, ferulic, 2,4-dimethoxybenzoic, and cinnamic acids were also reported (Cai et al., 2003). Valencia groundnut (var. fastiggiata) differing in seed color showed 34-fold differences in total phenols. Seeds with pink color had significantly higher levels of phenols than those with gray and yellow color. The major phenolic compounds in the testae of nearly all genotypes were p-coumaric and vanillic acids. Korean mung bean germplasm had 5-fold differences in total phenols. Kim et al. (2013) detected a total of 25 phenolic compounds in mung bean germplasm, with rutin being predominant, and its concentration among these germplasm varied from 1.09 to 2.72 mg g−1.
Table 4.
Germplasm-wide variations in phenylpropanoid constituents in food legumes.
| Germplasm (no.) | Variation in total phenols | References |
|---|---|---|
| Common bean (Phaseolus vulgaris) | ||
| Black, brown, pink, red, and white-grains (50) | 0.1–9.7 mg GAE g−1 DW | Agostini-Costa et al., 2015 |
| Landrace-based populations (10) | 0.007–0.032 mg GAE g−1 DW | Doria et al., 2012 |
| Varying in seed color and weight (29) | 6–14 mg g−1 GAE DW | Akond et al., 2011 |
| Wild and weedy types (64) | 50–131 mg kg−1 GAE fresh weight | Espinosa-Alonso et al., 2006 |
| Market types (15) | 0.19–0.48 mg g−1 GAE DW | Luthria and Pastor-Corrales, 2006 |
| Chickpea (Cicer arietinum L.) | ||
| Colored grains (17) | 0.2–32.6 mg catechin equiv. (CAE) g−1 DW; seed coat the major source of phenolics | Segev et al., 2010 |
| Cowpea (Vigna unguiculata (L.) Walp.) | ||
| Brown and white-grained (7) | 0.85–2.95 mg GAE g−1 DW | Noubissié et al., 2012 |
| Cultivars (17) | 0.35–3.77 mg g−1 DW | Cai et al., 2003 |
| Groundnut (Arachis hypogaea L.) | ||
| Gray, pink, purple, red, yellow, and variegated colored Valencia's (15) | Seed testa: 2.5–84.5 mg GAE g−1 DW; significantly greater phenols among accessions with pink grain color | Khaopha et al., 2012 |
| Mung bean (Vigna radiata (L.) R. Wilczek) | ||
| Germplasm (56) | 0.12–0.59 mg g−1 DW | Kim et al., 2013 |
| Soybean (Gylcine max (L.) Merr) | ||
| Black grains Japanese cultivars and landraces (227) | 75–380 and 19–389 mg GAE g−1 DW in two seasons; more phenols in purple flowers than white flowers producing cultivars | Phommalath et al., 2014 |
| Seed size variation (204) | 0.65–5.22 mg g−1 DW | Kim et al., 2012b |
| Black grains (60) | 5.12–60.58 mg GAE g−1 DW | Zhang et al., 2011 |
Original data on phenylpropanoid constituents given in papers cited here were converted and presented into mg g-1 dry weight.
Soybean germplasm from landraces and cultivars from China, Korea, and the USA showed 8–12-fold variations in total phenols. US soybean germplasm had higher mean total seed phenols (2.73 mg GAE g−1) than those from Korea (1.98 mg g−1) and China (1.680 mg g−1) (Kim et al., 2012b), as for black-grain Chinese germplasm (23.57 mg g−1; Zhang et al., 2011). Furthermore, the total phenols varied from 0.93 to 5.56 mg g−1 in North American soybean, 0.72 to 4.21 mg g−1 in Chinese soybean, 0.65 to 5.07 mg g−1 in Korean soybean (Kim et al., 2012b), 53 to 384 mg GAE g−1 in Japanese soybean (Phommalath et al., 2014) and 5.12 to 6.06 mg g−1 in black-grain Chinese germplasm (Zhang et al., 2011). Grain-size variations had significant effects on phenols, with higher mean total phenols in small- (2.24 mg g−1) as compared to medium- (1.93 mg g−1) and large- (1.95 mg g−1) grain types (Kim et al., 2012b). This suggests that phenolic compounds are condensed in small-grain soybean, whereas they appear diffused at lower densities in large-grain soybean (Kim et al., 2012b).
Phenolics in cereals
Cereal germplasm and cultivars have been the most extensively studied for variations in phenolics (Table 5). Two-fold to three-fold variations were noted for total phenols in barley. Catechine, p-coumaric, and ferulic acids were the most abundant (Dvořáková et al., 2008; Abdel-Aal et al., 2012; Gamel and Abdel-Aal, 2012). Genotypic differences for specific groups were also observed. For example, protocatechuic and caffeic acids were found in Egyptian hulled cultivars, but not in Canadian hulled cultivars (Gamel and Abdel-Aal, 2012). Blue-grain accessions had higher mean phenolics (7.73 mg g−1 DW) than white (6.69 mg g−1), purple (6.14 mg g−1), and black (5.61 mg g−1)-grain barley types (Siebenhandl-Ehn et al., 2011). Kim et al. (2007) also reported higher mean phenolics (0.27 mg g−1 DW) in blue and purple barley than in black-grained barley (0.21 mg g−1). Furthermore, a large range for total phenolics was noted within each group, which suggested that there are accessions with high phenolics in each color group (Siebenhandl-Ehn et al., 2011; Abdel-Aal et al., 2012).
Table 5.
Germplasm-wide variations in total phenylpropanoid constituents in staple cereals.
| Germplasm (no.) | Variation in total phenols | References |
|---|---|---|
| Barley (Hordeum vulgare L.) | ||
| Landraces (37) | 0.70–1.95 mg gallic acid equivalents (GAE) g−1 dry weight (DW) | Abidi et al., 2015 |
| Hulled and hull-less (11) | 0.06–0.14 mg GAE g−1 extract | Mahmoudi et al., 2015 |
| Colored grains (18) | 5.04–13.94; 7.97–14.12; 4.15–14.33; 8.20–8.94 mg g−1 GAE DW in black, blue, yellow, and mixed grain color, respectively | Abdel-Aal et al., 2012 |
| Two- and six-rows, hulled and hulless normal and waxy grains (6) | 171–554 mg g−1 DW | Gamel and Abdel-Aal, 2012 |
| Hulled and hulless cultivars (12) | 4.81–6.76 mg GAE g−1 DW | Holtekjølen et al., 2011 |
| Hulled and hulless cultivars (10) | 0.25–0.67 mg g−1 DW$ | Andersson et al., 2008 |
| Cultivars (10) | 0.25–0.49 mg GAE g−1 DW | Dvořáková et al., 2008 |
| Black, blue, purple grains (127) | 0.19–0.40 mg GAE g−1 DW; unhulled (0.27 g−1) > hulled (0.21 mg g−1); blue and purple (0.27 mg g−1)> black (0.21 mg g−1) | Kim et al., 2007 |
| Maize (Zea mays L.) | ||
| Blue-grain (7) | 10.10–13.47 mg GAE g−1 DW | Urias-Lugo et al., 2015 |
| Waxy (49) | 0.005–0.012 mg GAE g−1 DW | Harakotr et al., 2015 |
| Landrace populations (33) | 1.32–2.62 mg of GAE g−1 DW | González-Muñoz et al., 2013 |
| Inbred and landraces (10) | 5.23–10.53mg GAE g−1 DW | Žilić et al., 2012 |
| Red and blue colored grains (9) | 3.11–8.18 mg GAE g−1 DW | Montilla et al., 2011 |
| Waxy and normal yellow grains (4) | 0.23–3.88 mg GAE g−1 DW | Hu and Xu, 2011 |
| Colored and white grains (18) | 1.70–3.40 mg GAE g−1 DW | Lopez-Martinez et al., 2009 |
| Rice (Oryza sativa L.) | ||
| Diverse accessions (20) | 0.40–5.62 and 0.44–6.62 mg GAE g−1 DW in two seasons | Shao et al., 2014a |
| Black, red, and white grains (3) | 0.31–1.57 mg GAE g−1 DW | Shao et al., 2014b |
| Black and white grains (15) | 0.15–0.37 mg g−1 DW; greater variation in total soluble phenolics in black (0.17–0.37 mg g−1) than white (0.15–0.17 mg g−1) grains | Park et al., 2012 |
| Black, red, and white grains (6) | 1.40–11.87 mg GAE g−1 DW | Bordiga et al., 2014 |
| Cultivars with pigmented and non-pigmented grains (11) | 0.001–0.014 mg GAE g−1 bran; higher phenols in pigmented than non-pigmented; greater phenols in black-colored indica than black-colored japonica | Huang and Ng, 2012 |
| Black and red grains (13) | Black grains: 3.37–6.65 mg g−1 FAE DW; Red grains: 0.79–6.91 mg g−1 FAE DW | Sompong et al., 2011 |
| Black grains (12) | 23.65–73.67 mg GAE g−1 DW | Zhang et al., 2010 |
| Colored and white grains (21) | 1.07–4.25 mg FAE g−1 DW | de Mira et al., 2009 |
| Wild (11) | 2.47–4.07 mg FAE g−1 DW | Qiu et al., 2009 |
| White, red and black grains (481) | 1.08–1.24 mg GAE g−1 DW; black grains (10.56 mg) > red (4.70 mg) > white (1.52 mg) | Shen et al., 2009 |
| Rye (Secale cereale L.) | ||
| Cultivars (10) | 0.49–1.08 mg g−1 DW$ | Nyström et al., 2008 |
| Sorghum (Sorghum bicolor (L.) Conrad Moench) | ||
| Colored and white grains (381) | 2–14 mg GAE g−1 DW; proanthocyanidins high in brown while 3-deoxyanthocyanidins in red grains | Rhodes et al., 2014 |
| Colored and white grains (287) | 1–38 mg GAE g−1 DW; accessions with pigmented seeds had higher phenols | Dykes et al., 2014 |
| Lines and hybrids with black grains (8) | 5–20 mg GAE g−1 DW | Dykes et al., 2013 |
| Wheat (Triticum aestivum L.) | ||
| Black, purple, and white grains (4) | 0.51–0.66 mg GAE g−1 DW | Li et al., 2015 |
| Cultivars (23) | 2.90–5.65 mg GAE g−1 bran DW | Narwal et al., 2014 |
| Spelt (6) | 0.51–1.26 mg GAE g−1 DW | Gawlik-Dziki et al., 2012 |
| Hard and soft Canadian wheat cultivars (21) | Soluble and bound phenols, respectively, ranged from 0.11–0.15 and 0.80–1.07 mg g−1 DW | Ragaee et al., 2012 |
| Colored grains (13) | 120–177 mg FAE 100 g−1 DW; purple and blue grains had greater phenolic than red-grains | Eticha et al., 2011 |
| Market class (51) | 3.41–6.70 mg g−1 GAE DW | Verma et al., 2008 |
| Spring and winter wheat, spelt, durum, einkorn, emmer (175) | durum, spring, and winter wheat (0.61–0.70 mg FAE g−1); emmer (0.78 mg g−1)>einkorn (0.61 mg g−1)>Spelt (0.57 mg g−1); 2–3.6-fold variation within each group; winter wheat had greater variability (0.33–1.17 mg g−1) | Li et al., 2008 |
Original data on phenylpropanoid constituents given in papers cited here were converted and presented into mg g-1 dry weight.
total anthocyanin (not the anthocyanin compounds) value was given in the original literature.
Maize germplasm showed about 2-fold variation in total phenolics, except studies by Lopez-Martinez et al. (2009) and Hu and Xu (2011), who noted 16–20-fold variations. High phenolic content was found among Mexican landrace germplasm and in waxy corn germplasm from China. Black and purple grain showed higher levels of phenolics compared to red grain, while white kernels contained the lowest levels of phenolics (Lopez-Martinez et al., 2009; Hu and Xu, 2011; Montilla et al., 2011; González-Muñoz et al., 2013). Ferulic acid was the major phenolic, whereas p-coumaric, o-coumaric, vanillic, vanillin, and protocatechuic acids were found in significant quantities (Lopez-Martinez et al., 2009; Hu and Xu, 2011; Montilla et al., 2011; Žilić et al., 2012; González-Muñoz et al., 2013).
Total phenolics in rice germplasm showed up to 15-fold variation. One study involved 20 diverse rice accessions containing red and white grain types (Shao et al., 2014a) and another study had 481 accessions with black, red and white grain types (Shen et al., 2009); these showed large variability in total phenolics. Black rice germplasm contained higher levels of phenolics than red and white grain types, while white grain germplasm had the lowest levels of phenolics. Red grain types had higher levels of phenolics than white grain types, but lower levels than black grain types (Shen et al., 2009; Huang and Ng, 2012; Park et al., 2012; Shao et al., 2014a,b). Ferulic, p-coumaric, and salicylic acids were the major phenolic compounds (de Mira et al., 2009; Huang and Ng, 2012; Park et al., 2012), with pigmented rice grain containing greater soluble phenolic compounds than non-pigmented rice grain.
Wide range variations (7–38-fold) for total phenols were noted among sorghum germplasm (668 accessions) differing in grain color, while eight lines and hybrids showed 4-fold differences in phenols. High levels of total phenols were observed in pigmented genotypes (Dykes et al., 2014). Grain color had a significant effect on phenolic compounds. For example, red grain contained higher amounts of 3-deoxyanthocyanidins than brown or white grain, while brown grain contained significantly greater mean proanthocyanidins than red, white, or yellow grain (Rhodes et al., 2014). The grouping of sorghum germplasm based on biological and geographical origin also significantly affected total phenols and phenolic compounds. For example, bicolor and caudatum germplasm as a group had higher mean total phenols than durra and guinea, while bicolor and guinea-caudatum had higher mean proanthocyanidins and bicolor-durra and guinea-caudatum contained higher mean 3-deoxyanthocyanidins (Rhodes et al., 2014).
Wheat landraces and cultivars showed narrow variations (1.3–2.5-fold) for mean total phenols. Ferulic and p-coumaric acids were the major phenolics, while other compounds such as vanillic, syringic, 2,4-dihydroxybenzoic, and sinapic acids were also reported (Siebenhandl-Ehn et al., 2007; Li et al., 2008; Gawlik-Dziki et al., 2012; Ragaee et al., 2012). Some cultivar groups showed larger variability than others. For example, Li et al. (2008) showed greater range variations in winter wheat (3.6-fold; 0.33–1.17 mg g−1) than other cultivar groups (1.8–2.3-fold; spring: 0.46–0.89 mg g−1; durum: 0.54–1.08 mg g−1; spelt: 0.38–0.73 mg g−1; einkorn: 0.45–0.82 mg g−1; emmer: 0.51–1.16 mg g−1). Canadian “Western Red” spring wheat cultivars also showed greater variability in total phenols, with 4.62–6.70 mg g−1 in bran (Verma et al., 2008). Ancient cultivars showed significantly more phenolic compounds and isomer forms than modern wheat cultivars (Dinelli et al., 2009). However, ancient cultivars differed only slightly from modern wheats for most of the bioactive compounds (Laus et al., 2015; Shewry and Hey, 2015).
The key to successful crop improvement is a continued supply of genetic diversity that includes new or improved variability for target traits. Research toward the identification of health-promoting germplasm (i.e., containing more phenylpropanoids) is still in its infancy. Between 2009 and 2015, only a set of 4214 germplasm accessions of cereals and legumes were evaluated (Tables 1–5). Clearly, more investment is needed to identify germplasm that is rich in phenylpropanoids; screening of representative sets, such as core (Frankel, 1984) and mini core (Upadhyaya and Ortiz, 2001) subsets, will facilitate this task.
Developing elite germplasm/cultivars high in phenylpropanoids
Variation in seed color is associated with significant differences in phytochemicals. An indirect screening method that includes color parameters (i.e., L*, lightness; b*, yellowness; a*, redness; H*, hue angle) with an automatic color difference meter can be used for selecting phenylpropanoid-rich crops (Shen et al., 2009; Jaafar et al., 2013; Sharma et al., 2014).
Legumes
Soybean seeds are highly nutritious because they are rich in protein (>40%), oil (>18%), soluble carbohydrates (15%), and dietary fiber (15%). These seeds are exceptionally rich in isoflavones, such as genestein, daidzein, and glycetein, which are among the major components. High isoflavone cultivars are available in most soybean growing areas. The tofu (large-seeded) and the natto types (small-seeded, eaten as whole seeds) are grown in the USA and Canada (Anderson and Wolf, 1995). Soybean mutants modify seed chemistry based on single gene traits or by showing strong additive effects (Boerma and Specht, 2004). Combining genes to achieve the desired genotypes and capitalize on epistatic effects is being undertaken to improve this crop, both as food and feed (Wilson, 2012). Mutants enable genetic flexibility in tailoring soybean seed composition. The negative correlation between grain protein and isoflavone concentration might impose some biological constraint on the selection for these two beneficial traits (Murphy et al., 2009b). In contrast, similar relationships between isoflavone and linolenic (18:3) will be beneficial to the development of cultivars with high isoflavone and lower linolenic acid, the most undesirable trait (Wilson, 2012). The investment for crossbreeding soybean is smaller than that for transgenic breeding of this crop (Kumar and Ablett, 2010). Crossbred soybean is used for the improvement of transgenic soybean, because the gene base for crossbreeding is much wider than for transgenic breeding (Carter et al., 2004; Cui et al., 2004; Kumar and Ablett, 2010). It will be a challenge to maintain grain yield competitive soybean cultivars in a conventional background, as compared to transgenic soybean. This is likely to remain as a novel food until other traits are added to the trait profile of new cultivars. Despite these challenges, new cultivars with dramatic improvements in food and nutraceutical traits will emerge from global breeding programs. Soybean genomics will improve its breeding, which will greatly benefit human diets (Schmutz et al., 2010).
Cereals
Crossbreeding has led to cultivars and breeding populations rich in phenylpropanoids. For example, blue landraces in maize are a rich source of anthocyanins (Mendoza-Díaz et al., 2012). Highly productive blue maize hybrids adapted to subtropical environments or to Mexican highlands were bred with similar nutraceutical profiles to that of blue maize landraces (Urias-Peraldí et al., 2013; Urias-Lugo et al., 2015).
Some people prefer pigmented (red or black) rice grains due to their taste, texture, aroma, and ceremonial or medicinal value (Sweeney et al., 2006). Wickert et al. (2014) bred the cultivars “SCS119 Rubi” and “SCS120 Onix,” with red and black grains, respectively, for specialty rice markets in Brazil. These cultivars had the same protein, lipid, carbohydrate, mineral, and fiber content as white rice, but contained higher levels of anthocyanins and phenolics than their ancestor cultivars. The mean grain yields of these cultivars were only 78% of the best white grain control (9.8 t ha−1), which suggests that greater efforts are needed to raise the yield potential of such rice cultivars.
Red pericarp introgression lines (ILs) that originated from interspecific crosses showed several-fold differences in total phenolics, flavonoids, proanthocyanidins, and tannins in brown and milled rice fractions (Sharma et al., 2014). Furthermore, their yield and physiological grain traits were comparable to those of their respective recurrent parents. More recently, advanced breeding lines were evaluated for yield and phenylpropanoids in China. Several lines combined high phenolic, anthocyanin, and antioxidant capacities with high grain yield potential (Zhang et al., 2015).
Sorghum lines “Tx3362,” “ATx3363,” and “BTx3363” with black seed pericarp have been registered as elite lines that have very high levels of 3-deoxyanthocyanins. These lines can be used as seed parents to produce hybrids rich in 3-deoxyanthocyanins or as breeding lines to produce additional lines with these unique characteristics (Rooney et al., 2013a,b). The black-grained sorghum hybrid “ATx3363 × RTx3362” yielded 70–76% of red grain hybrids, but it contained exceptionally high levels of total phenols, tannins, and 3-deoxyanthocyanins (Rooney et al., 2013a). Although a set of six black-grained sorghum hybrids contained significantly higher concentrations of phenols, tannins and 3-deoxyanthocyanins across environments, the grain yield of the best hybrid (“A05029 × Tx3362”) was only 78% of the commercial white-grained hybrid (“ATx631 × RT- x- 436”; Hayes and Rooney, 2015). This low yield of black-grained hybrids showed that black grain is associated with some unknown factors that have a negative influence on grain yield.
The HEALTHGRAIN project in Europe clearly demonstrated substantial variations for bioactive compounds (e.g., alkylresorcinols, β-glucan, carotenoids, folates, phenolics, sterols, tocols), which are genetically determined, although environmental effects were also conspicuous (Ward et al., 2008; Shewry, 2009; Van der Kamp, 2012). Nevertheless it appears feasible to select for high levels of bioactive compounds, which will lead to a new generation of healthy cereals. Various genebank accessions, and advanced breeding lines and cultivars with blue or purple grain characteristics (e.g., “Amethyst,” “Indigo,” “Capo,” “Saturnus,” “Skorpion”) have been identified or crossbred in wheat (Zeven, 1991; Qualset et al., 2005; Zheng et al., 2009; Eticha et al., 2011; Guo et al., 2011; Jaafar et al., 2013; Martinek et al., 2014). There has been increased use of wheat genetic resources with different grain colors to develop blue- or purple-grain wheat cultivars (Martinek et al., 2014). Genetic research suggests that it is possible to increase the anthocyanin content using different genetic backgrounds for purple pericarp and blue-aleurone germplasm in wheat. The total anthocyanin content among breeding lines ranged from 0.018 to 0.298 mg g−1 (mean 0.13 mg g−1), and many lines contained anthocyanins in amounts ≥0.1 mg g−1 (Jaafar et al., 2013). The Crop Development Center at the University of Saskatchewan, Canada, bred the purple wheat cultivar “AnthoGrain™” that contained twice the anthocyanin content of earlier cultivars (http://www.agwest.sk.ca/blog/posts/wheat-of-the-future-might-be-purple.html). More recently, Gordeeva et al. (2015) bred near isogenic wheat lines (NILs) carrying various combinations of purple pericarp (Pp) alleles, using marker-aided back-crossing. These NILs represent a useful resource for studying the effects of grain pigmentation on other wheat traits.
A marker-aided recurrent backcrossing scheme using a high anthocyanin tropical maize line carrying regulatory genes (i.e., B1, Pl1) that are associated with anthocyanin production successfully converted traditional yellow popcorn into anthocyanin-rich popcorn (Lago et al., 2013, 2014). The popping ability, the expansion capacity of the kernel, the flake volume, and the taste preference of the bred cultivar were similar to the commercial yellow popcorn line.
Accessing consumer behavior to eating foods high in phenylpropanoids
Consumer acceptance of phytochemicals (such a flavonoids and phenols) in foods is widely recognized as a key factor for market orientation of new products. Bornkessel et al. (2011) noted that acceptance is mainly influenced by three factors: consumer characteristics, their purchasing power, and the product characteristics. Consumer characteristics concern personal health status and consumer awareness of phytochemicals as a special ingredient associated with certain additional benefits. The three main focus areas include consumer acceptance in general, health aspects and ingredients. The acceptance is determined by various factors such as primary health concerns, consumer familiarity with the new food concept and with the functional ingredients, nature of the product carrier, and communication and publicity of the health benefits. New product acceptance could be hindered because of the taste even though consumers perceive the benefits (Saba et al., 2010). Consumer knowledge of the health benefits of newly developed functional ingredients from phytochemicals appears to be relatively limited. Recent studies have shown that consumers of today are genuinely interested in products that are beneficial to their health. Middle-aged and elderly consumers tend to be substantially more health conscious than the younger generations. This is because they or members of their immediate social environment are much more likely to be diagnosed with a lifestyle-related disease (Nocella and Kennedy, 2012; Lähteenmäki, 2013).
The regulation of policies on health claims receives much attention worldwide because this helps consumers make informed healthy choices. Dean et al. (2007) researched public perceptions relating to different healthy grain-based foods (i.e., bread, pasta, biscuits) in Europe and how gender, nationality, type of food, health claims, and perceptions of the manufacturing processes influenced them. The results confirmed that perception of phytochemicals supplied by foods varies with gender, country, and differences in consumer perception of benefits relating to functional grain products. Men perceived more benefit in products with specific health claims than women did for products with general health claims. At the individual level, male level of perceived benefit in products with general health claims was, however, equally high as that of the women. Furthermore, modification of staple foods was perceived as being more beneficial than fun foods, and people preferred processes such as fortification and traditional cross-breeding to genetic modification. Lampila et al. (2009) investigated consumer perceptions of flavonoids using focus-group discussions in Europe. They noted that the average consumer was not familiar with the term “flavonoid.” However, consumer showed positive attitudes once informed about the beneficial effects of flavonoids on human health.
Consumer knowledge on the health effects of flavonoids is limited. Hence, there is need to improve marketing programs by including reliable nutritional information in food labels. The rising demand for such foods can be explained by the increasing cost of healthcare, the steady increase in life expectancy, and because older people wish to lead improved life quality during their “golden years” (Roberfroid, 2002; Siro et al., 2008). It should be noted that many industry-designed marketed nutritional supplements do not have beneficial effects for arresting the development of chronic diseases (such as cancer, cardiovascular diseases, diabetes, hypertension, inflammation, and obesity). This is partially due to the health effects resulting from a complex synergetic action of numerous phytochemicals supplied by foods or diets at nutritional doses and considering that a food is not a drug (Fardet and Rock, 2014).
Outlook
Consumers worldwide are becoming aware of the benefits of nutraceutical foods. A paradigm shift is gradually emerging for the development of nutritionally dense cultivars in addition to integrating genes for productivity and stress tolerance. As a result, nutrient-dense cultivars are being bred in cereals and legumes. Breeding for staple crops rich in phenylpropanoids is just beginning. A positive development is the search for phenylpropanoid-rich germplasm both in cereals and legumes that show broad variations in genebank germplasm. Many breeding programs are transferring this variation into the cultigen pool. As expected some yield penalty has been noted, which should be further investigated to assess cause-effect relationships to overcome the negative trade-off using cross-breeding or biotechnology facilitated genetic betterment. A systematic evaluation of germplasm using representative subsets is the ideal approach to discover new sources of variations for these compounds. An approach combining high throughput assays and wet chemistry should be integrated to support such breeding programs. The sensory attributes and consumer acceptance of flavonoid-rich staple foods should be further investigated for their wide acceptance. A food matrix-based approach instead of a reductionist approach is suggested with investigations into the effects of these compounds on human health.
Author contributions
SD and RO conceptualized the idea and finalized the outlines together with coauthors who contributed equally in manuscript writing and reading the full draft. RO, SD, and KS edited, while SD organized the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SD acknowledges the contribution of Ramesh Kotnana of Knowledge Sharing and Innovation Program of ICRISAT for arranging reprints on phytochemicals in cereals and legumes as a resource for drafting this manuscript. RO thanks the funding to SLU from PlantePigment—a project led by Chr. Hansen A/S with a grant from GUDP (Green Development and Demonstration Program, Denmark), while IC acknowledges funding from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01002202). SG acknowledges the funding to Research Nutriomics Chair (CAT-005) from Tecnologico de Monterrey, Escuela de Ingeniería y Ciencias. We are grateful to the two anonymous reviewer's for making useful suggestions on improving the manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00763
References
- Abdel-Aal E. S. M., Choo T. M. (2014). Differences in compositional properties of a hulless barley cultivar grown in 23 environments in eastern Canada. Can. J. Plant Sci. 94, 807–815. 10.4141/cjps2013-301 [DOI] [Google Scholar]
- Abdel-Aal E. M., Choo T.-M., Dhillon S., Rabalski I. (2012). Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 89, 198–204. 10.1094/CCHEM-10-11-0116 [DOI] [Google Scholar]
- Abdel-Aal E. M., Young J. C., Rabalski I. (2006). Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 54, 4696–4704. 10.1021/jf0606609 [DOI] [PubMed] [Google Scholar]
- Abidi I., Mansouri S., Radhouane L., Ksouri R., Felah M. E., Bouzid S. (2015). Phenolic, flavonoid and tannin contents of Tunisian barley landraces. Int. J. Agric. Innov. Res. 3, 1317–1323. [Google Scholar]
- Adom K. K., Sorrells M. E., Liu R. H. (2006). Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 53, 2297–2306. 10.1021/jf048456d [DOI] [PubMed] [Google Scholar]
- Afzal A., Gulzar I., Shahbaz M., Ashraf M. (2014). Water deficit-induced regulation ofgrowth, gas exchange, chlorophyll fluorescence, inorganic nutrient accumulation and antioxidative defense mechanism in mungbean (Vigna radiata (L.) Wilczek). J. Appl. Bot. Food Qual. 87, 147–156. 10.5073/JABFQ.2014.087.022 [DOI] [Google Scholar]
- Agati G., Brunetti C., Di Ferdinando M., Ferrini F., Pollastri S., Tattini M. (2013). Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol. Biochem. 72, 35–45. 10.1016/j.plaphy.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Agostini-Costa T. S., Teodoro A. F. P., Alves R. B. N., Braga L. R., Ribeiro I. F., Silva J. P., et al. (2015). Total phenolics, flavonoids, tannins and antioxidant activity of lima beans conserved in a Brazilian genebank. Ciência Rural Santa Maria 45, 335–341. 10.1590/0103-8478cr20140030 [DOI] [Google Scholar]
- Ahmed I. M., Cao F., Han Y., Nadira U. A., Zhang G., Wu F. (2013a). Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 141, 2743–2750. 10.1016/j.foodchem.2013.05.101 [DOI] [PubMed] [Google Scholar]
- Ahmed S., Faridi U., Shahid M., Nisar M. (2013b). Effect of phenolics in maize grains of different varieties on biology of Angoumois grain moth, Sitotroga cerealella (Oliv.) (Lepidoptera: Gelechiidae). Cereal Res. Commun. 41, 636–646. 10.1556/CRC.2013.0026 [DOI] [Google Scholar]
- Akond A. S. M. G. M., Khandaker L., Berthold J., Gates L., Peters K., Delong H., et al. (2011). Anthocyanin, total polyphenols and antioxidant activity of common bean. Am. J. Food Technol. 6, 385–394. 10.3923/ajft.2011.385.394 [DOI] [Google Scholar]
- Albert N. W. (2015). Subspecialization of R2R3-MYB repressors for anthocyanin and proanthocyanidin reduction in forage legumes. Front. Plant Sci. 6:1165 10.3389/fpls.2015.01165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert N. W., Lewis D. H., Zhang H., Schwinn K. E., Jameson P. E., Davies K. M. (2011). Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 65, 771–784. 10.1111/j.1365-313X.2010.04465.x [DOI] [PubMed] [Google Scholar]
- Ali Q., Ashraf M., Anwar F. (2010). Seed composition and seed oil antioxidant activity of maize under water stress. J. Am. Oil Chem. Soc. 87, 1179–1187. 10.1007/s11746-010-1599-5 [DOI] [Google Scholar]
- Andersson A. A. M., Lampi A.-M., Nyström L., Piironen V., Li L., Ward J. L., et al. (2008). Phytochemical and dietary fiber components in barley varieties in the health grain diversity screen. J. Agric. Food Chem. 56, 9767–9776. 10.1021/jf802037f [DOI] [PubMed] [Google Scholar]
- Anderson R. L., Wolf W. J. (1995). Compositional changes in trypsin inhibitors, phytic acid, and isoflavones related to soybean processing. J. Nutr. 125, 581S–588S. [DOI] [PubMed] [Google Scholar]
- Anton A. A., Lukow O. M., Fulcher R. G., Arntflied S. D. (2008). Influence of added bean flour (Phaseolus vugaris L.) and some physical and nutritional properties of wheat flour tortillas. Food Chem. 109, 33–41. 10.1016/j.foodchem.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Apak R., Gorinstein S., Böhm V., Schaich K. M., Özyürek M., Güçlü K. (2013). Methods of measurement and evaluation of natural antioxidant capacity activity (IUPAC Technical Report). Pure Appl. Chem. 85, 957–998. 10.1351/PAC-REP-12-07-15 [DOI] [Google Scholar]
- Bai Y., Pattanaik S., Patra B., Werkman J. R., Xie C. H., Yuan L. (2011). Flavonoid-related basic helix–loop–helix regulators, NtAn1a and NtAn1b, of tobacco have originated from two ancestors and are functionally active. Planta 234, 363–375. 10.1007/s00425-011-1407-y [DOI] [PubMed] [Google Scholar]
- Bamisile B. S., Adesina J. M., Ofuya T. I. (2014). Relative susceptibility and proximate composition of some imported and local rice varieties to infestation and damage by Sitophilus oryzae L. (Coleoptera: Curculionidae). Mol. Entomol. 5, 18–29. 10.5376/me.2014.05.0003 [DOI] [Google Scholar]
- Ban Y., Honda C., Hatsuyama Y., Igarashi M., Bessho H., Moriguchi T. (2007). Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 48, 958–970. 10.1093/pcp/pcm066 [DOI] [PubMed] [Google Scholar]
- Bao J. S., Cai Y. Z., Corke H. (2001). Prediction of rice starch quality parameters by near infrared reflectance spectroscopy. J. Food Sci. 66, 936–939. 10.1111/j.1365-2621.2001.tb08215.x [DOI] [Google Scholar]
- Bao J. S., Wang Y. F., Shen Y. (2007). Determination of apparent amylose content, pasting properties and gel texture of rice starch by near infrared spectroscopy. J. Sci. Food Agric. 87, 2040–2048. 10.1002/jsfa.2960 [DOI] [Google Scholar]
- Baudry A., Heim M. A., Dubreucq B., Caboche M., Weisshaar B., Lepiniec L. (2004). TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 39, 366–380. 10.1111/j.1365-313X.2004.02138.x [DOI] [PubMed] [Google Scholar]
- Begum A., Goswami A., Chowdhury P. (2015). A comparative study on free and bound phenolic acid content and their antioxidant activity in bran of rice (Oryza sativa L.) cultivars of Eastern Himalayan range. Int. J. Food Sci. Technol. 50, 2529–2536. 10.1111/ijfs.12920 [DOI] [Google Scholar]
- Bertoia M. L., Rimm E. B., Mukamal K. J., Hu F. B., Willett W. C., Cassidy A. (2015). Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124 086 US men and women followed for up to 24 years. Br. Med. J. 352, i17. 10.1136/bmj.i17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beta T., Rooney L. W., Marovatsanga L. T., Taylor J. R. N. (1999). Phenolic compounds and kernel characteristics of Zimbabwean sorghums. J. Sci. Food Agric. 79, 1003–1010. [Google Scholar]
- Bi Y., Li W., Xiao J., Lin H., Liu M., Luan X., et al. (2015). Heterosis and combining ability estimates in isoflavone content using different parental soybean accessions: wild soybean, a valuable germplasm for soybean breeding. PLoS ONE 10:e0114827. 10.1371/journal.pone.0114827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner L. K., Schoenbichler S. A., Bonn G. K., Huck C. W. (2013). Near infrared spectroscopy (NIRS) as a tool to analyze phenolic compounds in plants. Curr. Anal. Chem. 9, 417–423. 10.2174/1573411011309030010 [DOI] [Google Scholar]
- Blankenberg S., Rupprecht H. J., Bickel C., Peetz D., Hafner G., Tiret L., et al. (2001). Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 104, 1336–1342. 10.1161/hc3701.095949 [DOI] [PubMed] [Google Scholar]
- Bobo-García G., Davidov-Pardo G., Arroqui C., Vírseda P., Marín-Arroyo M. R., Navarro M. (2015). Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 95, 204–209. 10.1002/jsfa.6706 [DOI] [PubMed] [Google Scholar]
- Boerma H. R., Specht J. E. (eds.). (2004). Soybeans: Improvement, Production and Uses. 3rd Edn. Agron. Monogr. Vo.l 16. Madison, WI: ASA, CSSA, and SSSA. [Google Scholar]
- Bogs J., Downey M. O., Harvey J. S., Ashton A. R., Tanner G. J., Robinson S. P. (2005). Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. Biochem. 139, 652–663. 10.1104/pp.105.064238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonina F., Lanza M., Montenegro L., Puglisi C., Tomaino A., Trombetta D., et al. (1996). Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 145, 87–94. 10.1016/S0378-5173(96)04728-X [DOI] [Google Scholar]
- Bordiga M., Gomez-Alonso S., Locatelli M., Travaglia F., Coïsson J. D., Hermosin-Guiterrez I., et al. (2014). Phenolics characterization and antioxidant activity of six different pigmented Oryza sativa cultivars grown in Piedmong (Italy). Food Res. Int. 65, 282–290. 10.1016/j.foodres.2014.03.007 [DOI] [Google Scholar]
- Bornkessel S., Bröring S., Omta S. W. F. (2011). Consumer acceptance of functional foods and their ingredients-positioning options for innovations on the borderline between foods and drugs, in International Food and Agribusiness Management Association. 21st Annual World Symposium (Frankfurt: ). [Google Scholar]
- Boyle S. P., Dobson V. L., Duthie S. J., Hinselwood D. C., Kyle J. A. M., Collins A. R. (2000). Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur. J. Clin. Nutr. 54, 774–782. 10.1038/sj.ejcn.1601090 [DOI] [PubMed] [Google Scholar]
- Bravo L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56, 317–333. 10.1111/j.1753-4887.1998.tb01670.x [DOI] [PubMed] [Google Scholar]
- Budevska B. O. (2002). Vibrational Spectroscopy Imaging of Agricultural Products. New York, NY: John Wiley and Sons, Ltd. [Google Scholar]
- Bunaciu A. A., Aboul-Enein H. Y., Fleschin S. (2012). FTIR spectrophotometric methods used for antioxidant activity assay in medicinal plants. Appl. Spectrosc. Rev. 47, 245–255. 10.1080/05704928.2011.645260 [DOI] [Google Scholar]
- Burešováet V., Kopecký D., Bartoš J., Martinek P., Watanabe N., Vyhnánek T., et al. (2015). Variation in genome composition of blue-aleurone wheat. Theor. Appl. Genet. 128, 273–282. 10.1007/s00122-014-2427-3 [DOI] [PubMed] [Google Scholar]
- Bustos D. V., Riegel R., Calderini D. F. (2012). Anthocyanin content of grains in purple wheat is affected by grain position, assimilate availability and agronomic management. J. Cereal Sci. 55, 257–264. 10.1016/j.jcs.2011.12.001 [DOI] [Google Scholar]
- Byrne P. F., McMullen M. D., Snook M. E., Musket T. A., Theuri J. M., Windstrom N. W., et al. (1996). Quantitative trait loci and metabolic pathways: genetic control of the concentration of maysin, a corn earworm resistance factor, in maize silks. Proc. Natl. Acad. Sci. U.S.A. 93, 8820–8825. 10.1073/pnas.93.17.8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R., Hettiarachchy N. S., Jalaluddin M. (2003). High performance of liquid chromatography determination of phenolic constituents in 17 varieties of cowpeas. J. Agric. Food Chem. 51, 1623–1627. 10.1021/jf020867b [DOI] [PubMed] [Google Scholar]
- Caldwell C. R., Britz S. J., Mirecki R. M. (2005). Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean [Glycine max (L.) Merrill] grown in controlled environments. J. Agric. Food Chem. 53, 1125–1129. 10.1021/jf0355351 [DOI] [PubMed] [Google Scholar]
- Carey C. C., Strahle J. T., Selinger D. A., Chandler V. L. (2004). Mutations in the pale aleurone color1 regulatory gene of the Zea mays anthocyanin pathway have distinct phenotypes relative to the functionally similar TRANSPARENT TESTA GLABRA1 gene in Arabidopsis thaliana. Plant Cell 16, 450–464. 10.1105/tpc.018796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera C. S., Dardanelli J. L. (2015). Changes in the relationship between temperature during the seed-filling period and soybean seed isoflavones under water-deficit conditions. J. Agron. Crop Sci. 10.1111/jac.12147 [DOI] [Google Scholar]
- Carrera C. S., Dardanelli J. L., Soldini D. O. (2014). Genotypic and environmental variation in seed nutraceutical and industrial composition of non-transgenic soybean (Glycine max) genotypes. Crop Pasture Sci. 65, 1311–1322. 10.1071/cp14114 [DOI] [Google Scholar]
- Carter T. E., Jr., Nelson R. L., Sneller C. H., Cui Z. (2004). Genetic diversity in soybeans in The Soybeans: Improvement, Production and Uses, Agron. Monogr. Vol. 16, 3rd Edn., eds Boerma H. R., Specht J. E. (Madison, WI: ASA, CSSA, and SSSA; ), 303–416. [Google Scholar]
- Casas M. I., Duarte S., Doseff A. I., Grotewold E. (2014). Flavone-rich maize: an opportunity to improve the nutritional value of an important commodity crop. Front. Plant Sci. 5:440. 10.3389/fpls.2014.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. M., Jemal A., Ward E. (2009). International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 18, 1688–1694. 10.1158/1055-9965.EPI-09-0090 [DOI] [PubMed] [Google Scholar]
- Cercamondi C. I., Egli I. M., Zeder C., Hurrell R. F. (2014). Sodium iron edta and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br. J. Nutr. 111, 481–489. 10.1017/S0007114513002705 [DOI] [PubMed] [Google Scholar]
- Chakraborty U., Pradhan B. (2012). Drought stress-induced oxidative stress and antioxidative responses in four wheat (Triticum aestivum L.) varieties. Arch. Agron. Soil Sci. 58, 617–630. 10.1080/03650340.2010.533660 [DOI] [Google Scholar]
- Chandler V. L., Radicella J. P., Robbins T. P., Chen J., Turks D. (1989). Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell 1, 1175–1183. 10.1105/tpc.1.12.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N., Agrawal S. B. (2015). The role of elevated ozone on growth, yield and seed quality amongst six cultivars of mung bean. Ecotoxicol. Environ. Safety 111, 286–294. 10.1016/j.ecoenv.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Chavez-Santoscoy R. A., Gutierrez-Uribe J. A., Granados O., Torre-Villalvazo I., Tovar A. R., Serna-Saldivar S. O., et al. (2014). Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 112, 886–899. 10.1017/S0007114514001536 [DOI] [PubMed] [Google Scholar]
- Chavez-Santoscoy R. A., Gutierrez-Uribe J. A., Serna-Saldívar S. O. (2013). Effect of flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats as cholesterol micelle disruptors. Plant Foods Hum. Nutr. 68, 416–423. 10.1007/s11130-013-0384-7 [DOI] [PubMed] [Google Scholar]
- Chavez-Santoscoy R. A., Gutierrez-Uribe J. A., Serna-Saldívar S. O., Perez-Carrilo E. (2016). Production of maize tortillas and cookies from nixtamalized flour enriched with anthocyanins, flavonoids and saponins extracted from black bean (Phaseolus vulgaris) seed coat. Food Chem. 192, 90–97. 10.1016/j.foodchem.2015.06.113 [DOI] [PubMed] [Google Scholar]
- Chen X. Q., Nagao N., Itani T., Irifune K. (2012). Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different colored rice. Food Chem. 135, 2783–2788. 10.1016/j.foodchem.2012.06.098 [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhao J., Liu M., Cai J., Liu J. (2008). Determination of total phenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J. Pharm. Biomed. Anal. 46, 568–573. 10.1016/j.jpba.2007.10.031 [DOI] [PubMed] [Google Scholar]
- Cheng Z., Moore J., Yu L. (2006). High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 54, 7429–7436. 10.1021/jf0611668 [DOI] [PubMed] [Google Scholar]
- Chennupati P., Seguin P., Liu W. (2011). Effects of high temperature stress at different stages on soybean isoflavone and tocopherol concentrations. J. Food Agric. Chem. 59, 13081–13088. 10.1021/jf2037714 [DOI] [PubMed] [Google Scholar]
- Cheynier V., Comte G., Davies K. M., Lattanzio V., Martens S. (2013). Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 72, 1–20. 10.1016/j.plaphy.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Chiremba C., Taylor J. R. N., Rooney L. W., Beta T. (2012). Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chem. 134, 81–88. 10.1016/j.foodchem.2012.02.067 [DOI] [PubMed] [Google Scholar]
- Choung M.-G., Choi B.-R., An Y.-N., Chu Y.-C., Cho Y.-S. (2003). Anthocyanin profiles of Korean cultivated kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 51, 7040–7043. 10.1021/jf0304021 [DOI] [PubMed] [Google Scholar]
- Chunthaburee S., Sanitchon J., Pattanagul W., Theerakulpisut P. (2015). Effects of salt stress after late booting stage on yield and antioxidant capacity in pigmented rice grains and alleviation of the salt-induced yield reduction by exogenous spermidine. Plant Prod. Sci. 18, 32–42. 10.1626/pps.18.32 [DOI] [Google Scholar]
- Codgill R. P., Hurburgh C. R., Jr., Jensen T. C., Jones R. W. (2002). Single kernel maize analysis by near-infrared hyperspectral imaging, in Proc. 10th Int. Conf. on Near-infrared Spectroscopy, eds Davies A. M. C., Cho R. K. (Chichester: NIR publications; ), 243–247. [Google Scholar]
- Codgill R. P., Hurburgh C. R., Jr., Rippke G. R. (2004). Single kernel maize analysis by near-infrared hyperspectral imaging. T. ASABE 47, 311–320. [Google Scholar]
- Cook N. C., Samman S. (1996). Flavonoid chemistry, metabolism, cardioprotective effects and dietary sources. J. Nutr. Biochem. 7, 66–76. 10.1016/0955-2863(95)00168-9 [DOI] [Google Scholar]
- Corcoran M. P., McKay D. L., Blumberg J. B. (2012). Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J. Nutr. Gerontol. Geriatr. 31, 176–189. 10.1080/21551197.2012.698219 [DOI] [PubMed] [Google Scholar]
- Cozzolino D. (2015). The role of visible and infrared spectroscopy combined with chemometrics to measure phenolic compounds in grape and wine samples. Molecules 20, 726–735. 10.3390/molecules20010726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino D., Cynkar W. U., Dambergs R. G., Mercurio M. D., Smith P. A. (2008). Measurement of condensed tannins and dry matter in red grape homogenates using near infrared spectroscopy and partial least squares. J. Agric. Food Chem. 56, 7631–7636. 10.1021/jf801563z [DOI] [PubMed] [Google Scholar]
- Cozzolino D., Kwiatkowski M. J., Parker M., Cynkar W. U., Dambers R. G., Gishen M., et al. (2004). Prediction of phenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal. Chim. Acta 513, 73–80. 10.1016/j.aca.2003.08.066 [DOI] [Google Scholar]
- Cui Z., James A. T., Miyazaki S., Wilson R. F., Carter T. E., Jr. (2004). Breeding specialty soybeans for traditional and new soy foods in The Soybeans as Functional Foods and Ingredients, ed Liu K. S. (Champaign, IL: Am. Oil Chem. Soc. Press; ), 264–322. [Google Scholar]
- Curtis P. J., Kroon P. A., Hoollands W. J., Walls R., Jenkins G., Kay C. D., et al. (2009). Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting in elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 139, 2266–2671. 10.3945/jn.109.113126 [DOI] [PubMed] [Google Scholar]
- Das P. K., Shin D. H., Choi S. B., Yoo S. D., Choi G., Park Y. I. (2012). Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol. Cells. 34, 93–101. 10.1007/s10059-012-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Shepherd R., Aryola A., Vassallo M., Winkelmann M., Claupein E., et al. (2007). Consumer perceptions of healthy cereal products and production methods. J. Cereal Sci. 46, 188–196. 10.1016/j.jcs.2007.06.007 [DOI] [Google Scholar]
- de Lima P. F., Colombo C. A., Chiorato A. F., Yamaguchi L. F., Kato M. J., Carbonell S. A. M. (2014). Occurrence of flavonoids in Brazilian common bean germplasm (Phaseolus vulgaris L.). J. Agric. Food Chem. 62, 9699–9704. 10.1021/jf5033312 [DOI] [PubMed] [Google Scholar]
- de Leonardis A. M., Fragasso M., Beleggia R., Ficco D. B. M., de Vita P., Mastrangelo A. M. (2015). Effect of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int. J. Mol. Sci. 16, 30382–30404. 10.3390/ijms161226241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira L. L., de Carvalho M. V., Melo L. (2014). Health promoting and sensory properties of phenolic compounds in food. Rev. Ceres Viçosa 61, 764–779. 10.1590/0034-737x201461000002 [DOI] [Google Scholar]
- Deluc L., Barrieu F., Marchive C., Lauvergeat V., Decendit A., Richard T., et al. (2006). Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 140, 499–511. 10.1104/pp.105.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mira N. V. M., Massaretto I. L., Pascual C. M. C. I., Marquez U. M. L. (2009). Comparative study of phenolic compounds in different Brazilian rice (Oryza sativa L.) genotypes. J. Food Comp. Anal. 22, 405–409. 10.1016/j.jfca.2008.06.012 [DOI] [Google Scholar]
- Dias A. S., Lidon F. C. (2010). Bread and durum wheat tolerance under heat stress: a synoptical overview. J. Food Agric. 22, 412–436. 10.9755/ejfa.v22i6.4660 [DOI] [Google Scholar]
- Diczházi I., Kursinszki L. (2014). Anthocyanin content and composition in winter blue barley cultivars and lines. Cereal Chem. 91, 195–200. 10.1094/CCHEM-05-13-0091-R [DOI] [Google Scholar]
- Dinelli G., Carretero A. S., Di Silvestro R., Marotti I., Fu S., Benedettelli S., et al. (2009). Determination of phenolic compounds in modern and old varieties of durum wheat using liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 1216, 7229–7240. 10.1016/j.chroma.2009.08.041 [DOI] [PubMed] [Google Scholar]
- Dlamini N. R., Taylor J. R. N., Rooney L. W. (2007). The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chem. 105, 1412–1419. 10.1016/j.foodchem.2007.05.017 [DOI] [Google Scholar]
- Dobrovolskaya O., Arbuzova V. S., Lohwasser U., Röder M. S., Börner A. (2006). Microsatellite mapping of complementary genes for purple grain color in bread wheat (Triticum aestivum L.). Euphytica 150, 355–364. 10.1007/s10681-006-9122-7 [DOI] [Google Scholar]
- Doria E., Campion B., Sparvooli F., Tava A., Nielsen E. (2012). Anti-nutrient components and metabolites with health implications in seeds of 10 common bean (Phaseolus vulgaris L. and P. lunatus L.) landraces cultivated in southern Italy. J. Food Comp. Anal. 26, 72–80. 10.1016/j.jfca.2012.03.005 [DOI] [Google Scholar]
- Dubcovsky J., Luo M. C., Zhong G. Y., Bransteitter R., Desai A., Kilian A., et al. (1996). Genetic map of diploid wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143, 983–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C., Le Gourrierec J., Baudry A., Huep G., Lanet E., Debeaujon I., et al. (2008). MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 55, 940–953. 10.1111/j.1365-313X.2008.03564.x [DOI] [PubMed] [Google Scholar]
- Dvořáková M., Guido L. F., Dostálek P., Skulilová Z., Moreira M. M., Barros A. A. (2008). Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 114, 27–33. 10.1002/j.2050-0416.2008.tb00302.x [DOI] [Google Scholar]
- Dwivedi S., Sahrawat K. L., Rai K. N., Blair M. W., Anderson M. S., Pfeiffer W. (2012). Nutritionally enhanced staple food crops. Plant Breed. Rev. 36, 169–291. 10.1002/9781118358566.ch3 [DOI] [Google Scholar]
- Dykes L., Hoffmann L., Portillo-Rodriguez O., Rooney W. L., Rooney L. W. (2014). Prediction of total phenols, condensed tannins, and 3-deoxyanthocyanidins in sorghum grain using near-infrared (NIR) spectroscopy. J. Cereal Sci. 60, 138–142. 10.1016/j.jcs.2014.02.002 [DOI] [Google Scholar]
- Dykes L., Rooney W. L., Rooney L. W. (2013). Evaluation of phenolics and antioxidant activity of black sorghum. J. Cereal Sci. 58, 278–283. 10.1016/j.jcs.2013.06.006 [DOI] [Google Scholar]
- Dykes L., Rooney L. W., Waniska R. D., Rooney W. L. (2005). Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 53, 6813–6818. 10.1021/jf050419e [DOI] [PubMed] [Google Scholar]
- Dykes L., Seitz L. M., Rooney W. L., Rooney L. W. (2009). Flavonoid composition of red sorghum genotypes. Food Chem. 116, 313–317. 10.1016/j.foodchem.2009.02.052 [DOI] [PubMed] [Google Scholar]
- Eggert K., Hollmann J., Hiller B., Kruse H. P., Rawel H. M., Pawelzik E. (2010). Effects of fusarium infection on the phenolics in emmer and naked barley. J. Agric. Food Chem. 58, 3043–3049. 10.1021/jf903545j [DOI] [PubMed] [Google Scholar]
- Espinosa-Alonso L. G., Lygin A., Widholm J. M., Valverde M. E., Paredes-Lopez O. (2006). Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 54, 4436–4444. 10.1021/jf060185e [DOI] [PubMed] [Google Scholar]
- Espley R. V., Hellens R. P., Putterill J., Stevenson D. E., Kutty-Amma S., Allan A. C. (2007). Red coloration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 49, 414–427. 10.1111/j.1365-313X.2006.02964.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha F., Grausgruber H., Siebenhandl-Ehn S., Berghofer E. (2011). Some agronomic and chemical traits of blue aleurone and purple pericarp wheat (Triticum L.). J. Agric. Sci. Technol. B 1, 48–58. [Google Scholar]
- Evans P., Halliwell B. (2001). Micronutrients: oxidant/antioxidant status. Br. J. Nutr. 85, S67–S74. 10.1079/bjn2000296 [DOI] [PubMed] [Google Scholar]
- Fardet A., Rock E. (2014). The search for a new paradigm to study micronutrient and phytochemical bioavailability: from reductionism to holism. Med. Hypoth. 82, 181–186. 10.1016/j.mehy.2013.11.035 [DOI] [PubMed] [Google Scholar]
- Fernandez-Orozco R., Li L., Harflett C., Shewry P. R., Ward J. L. (2010). Effects of environment and genotype on phenolic acids in wheat in the healthgrain diversity screen. J. Agric. Food Chem. 58, 9341–9352. 10.1021/jf102017s [DOI] [PubMed] [Google Scholar]
- Ferreyra M. L. F., Rius S. P., Casati P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3:222. 10.3389/fpls.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficco D. B. M., de Simone V., Colecchia S. A., Pecorella I., Platani C., Nigro F., et al. (2014). Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (T. turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 62, 8686–8695. 10.1021/jf5003683 [DOI] [PubMed] [Google Scholar]
- Finocchiaro F., Ferrari B., Gianinetti A., Dall'Asta C., Galaverna G., Scazzina F., et al. (2007). Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 51, 1006–1019. 10.1002/mnfr.200700011 [DOI] [PubMed] [Google Scholar]
- Fogarasi A. L., Kun S., Tankó G., Stefanovits-Bányai É., Hegyesné-Vecseri B. (2015). A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 167, 1–6. 10.1016/j.foodchem.2014.06.084 [DOI] [PubMed] [Google Scholar]
- Fox G., Manley M. (2014). Applications of single kernel conventional and hyperspectral imaging near infrared spectroscopy in cereals. J. Sci. Food Agric. 94, 174–179. 10.1002/jsfa.6367 [DOI] [PubMed] [Google Scholar]
- Frankel O. H. (1984). Genetic perspective of germplasm collection in The Genetic Manipulations: Impact on Man and Society, eds Arber W., Limensee K., Peacock W. J., Stralinger P. (Cambridge: Cambridge University Press; ), 161–170. [Google Scholar]
- Furbank R. T., Tester M. (2011). Phenomics technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 16, 635–644. 10.1016/j.tplants.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Galland M., Boutet-Mercey S., Lounifi I., Godin B., Belzergue S., Grandjean O., et al. (2014). Compartmentation and dynamics of flavone metabolism in dry and germinated rice seeds. Plant Cell Physiol. 55, 1646–1659. 10.1093/pcp/pcu095 [DOI] [PubMed] [Google Scholar]
- Gamel T. H., Abdel-Aal E. M. (2012). Phenolic acids and antioxidant properties of barley whole grain and pearling fractions. Agric. Food Sci. 21, 118–131. [Google Scholar]
- García-Lara S., Bergvinson D. J. (2014). Phytochemical changes during recurrent selection for storage pest resistance in tropical maize. Crop Sci. 54, 2423–2432. 10.2135/cropsci2014.03.0223 [DOI] [Google Scholar]
- Gawlik-Dziki U., Świeca M., Dziki D. (2012). Comparison of phenolic acids profile and antioxidant potential of six varieties of Spelt (Triticum spelta L.). J. Agric. Food Chem. 60, 4603–4612. 10.1021/jf3011239 [DOI] [PubMed] [Google Scholar]
- González-Muñoz A., Quesille-Villalobos A. M., Fuentealba C., Shetty K., Ranilla L. G. (2013). Potential of Chilean native corn (Zea mays L.) accessions as natural sources of phenolic antioxidants and in vitro bioactivity for hyperglycemia and hypertension management. J. Agric. Food Chem. 61, 10995–11007. 10.1021/jf403237p [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Zhao M., Leavitt J. M., Lloyd A. M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 53, 814–827. 10.1111/j.1365-313X.2007.03373.x [DOI] [PubMed] [Google Scholar]
- Gordeeva E. I., Shoeva O. Y., Khlestkina E. K. (2015). Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 203, 469–476. 10.1007/s10681-014-1317-8 [DOI] [Google Scholar]
- Gou J. Y., Felippes F. F., Liu C. J., Weigel D., Wang J. W. (2011). Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23, 1512–1522. 10.1105/tpc.111.084525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goufo P., Pereira J., Figeiredo N., Oliveira M. B. P. P., Carranca C., Rosa E. A. S., et al. (2014). Effect of elevated carbon dioxide (CO2) on phenolic acids, flavonoids, tocopherols, tocotrienols, γ-oryzanol and antioxidant capacities of rice (Oryza sativa L.). J. Cereal Sci. 59, 15–24. 10.1016/j.jcs.2013.10.013 [DOI] [Google Scholar]
- Goufo P., Trindade H. (2014). Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ oryzanol and phytic acid. Food Sci. Nutr. 2, 75–104. 10.1002/fsn3.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf B., Milbury P., Blumberg J. (2005). Flavonols, flavones, flavanones, and human health: epidemiological evidence. J. Med. Food, 8, 281–290. 10.1089/jmf.2005.8.281 [DOI] [PubMed] [Google Scholar]
- Graham H. D. (1992). Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food chem. 40, 801–805. 10.1021/jf00017a018 [DOI] [Google Scholar]
- Grieger J. A., Haas J. D., Murray-Kolb L. E., Kris-Etherton P., Beard J. L. (2008). Nutrient adequacy and food group consumption of Filipino novices and religious sisters over a nine month period. Asia Pac. J. Clin. Nutr. 17, 566–572. [PubMed] [Google Scholar]
- Grotewold E., Sainz M. B., Tagliani L., Hernandez J. M., Bowen B., Chandler V. L. (2000). Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc. Natl. Acad. Sci. U.S.A. 97, 13579–13584. 10.1073/pnas.250379897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. Y., Foley M. E., Horvath D. P., Anderson J. V., Feng J., Zhang L., et al. (2011). Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 189, 1515–1524. 10.1534/genetics.111.131169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guajardo-Flores D., Rempel C., Gutiérrez-Uribe J. A., Serna-Saldívar S. O. (2015). Influence of excipients and spray drying on the physical and chemical properties of nutraceutical capsules containing phytochemicals from black bean extract. Molecules 20, 21626–21635. 10.3390/molecules201219792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Xu P., Zhang Z., Wang D., Jin M., Teng A. (2011). Segregation ratio of coloured grains in crossed wheat. Australian J. Crop Sci. 5, 589–594. [Google Scholar]
- Gutierrez-Gonzalez J. J., Vuong T. D., Zhong R., Yu O., Lee J. D., Shannon G., et al. (2011). Major locus and other novel additive and epistatic loci involved in modulation of isoflavone concentration in soybean seeds. Theor. Appl. Genet. 123, 1375–1385. 10.1007/s00122-011-1673-x [DOI] [PubMed] [Google Scholar]
- Gutierrez-Gonzalez J. J., Wu X., Gillman J. D., Lee J.-D., Yu O., Shannon G., et al. (2010). Intricate environment-modulated genetic networks control isoflavone accumulation in soybean seeds. BMC Plant Biol. 10:105. 10.1186/1471-2229-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Gonzalez J. J., Wu X., Zhang J., Lee J. D., Ellersieck M., Shannon J. G., et al. (2009). Genetic control of soybean seed isoflavone content: importance of statistical model and epistasis in complex traits. Theor. Appl. Genet. 119, 1069–1083. 10.1007/s00122-009-1109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C., Oliver Y., Deyue Y. (2008). Polymorphisms of IFS1 and IFS2 gene are associated with isoflavone concentrations in soybean seeds. Plant Sci. 175, 505–512. 10.1016/j.plantsci.2008.05.020 [DOI] [Google Scholar]
- Harakotr B., Suriharn B., Scott M. P., Lertrot K. (2015). Genotypic variability in anthocyanins, total phenolics, and antioxidant activity among diverse waxy corn germplasm. Euphytica 203, 237–248. 10.1007/s10681-014-1240-z [DOI] [Google Scholar]
- Hart J. J., Tako E., Kochian L. V., Glahn R. P. (2015). Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 63, 5950–5956. 10.1021/acs.jafc.5b00531 [DOI] [PubMed] [Google Scholar]
- Hassan H., Fan M., Zhang T., Yang K. (2015). Prediction of total phenolics and flavonoids contents in Chinese wild rice (Zizania latifolia) using FT-NIR spectroscopy. Am. J. Food Technol. 10, 109–117. 10.3923/ajft.2015.109.117 [DOI] [Google Scholar]
- Havrlentová M., Pšenáková I., Žofajová A., Rückschloss L'., Kraic J. (2014). Anthocyanins in wheat seed–A mini review. Nova Biotechnol. Chim. 13, 1–12. 10.2478/nbec-2014-0001 [DOI] [Google Scholar]
- Hayes C. M., Rooney W. L. (2015). Agronomic performance and heterosis of specialty grain sorghum hybrids with a black pericarp. Euphytica 196, 459–466. 10.1007/s10681-013-1047-3 [DOI] [Google Scholar]
- He J., Giusti M. (2010). Anthocyanins: natural colorants with health promoting properties. Ann. Rev. Food Sci. Technol. 1, 163–187. 10.1146/annurev.food.080708.100754 [DOI] [PubMed] [Google Scholar]
- Herald T. J., Gadgil P., Perumal R., Bean S. R., Wilson J. D. (2014). High-throughput micro-plate HCl–vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 94, 2133–2136. 10.1002/jsfa.6538 [DOI] [PubMed] [Google Scholar]
- Herald T. J., Gadgil P., Tilley M. (2012). High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 92, 2326–2331. 10.1002/jsfa.5633 [DOI] [PubMed] [Google Scholar]
- Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V. (2011). Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 62, 2465–2483. 10.1093/jxb/erq442 [DOI] [PubMed] [Google Scholar]
- Hichri I., Heppel S. C., Pillet J., Leon C., Czemmel S., Delrot S., et al. (2010). The basic helix-loop-helix transcription factor myc1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant 3, 509–523. 10.1093/mp/ssp118 [DOI] [PubMed] [Google Scholar]
- Himi E., Maekawa M., Noda K. (2011). Differential expression of three flavanone 3-hydroxylase genes in grains and coleoptiles of wheat. Int. J. Plant Genom. 2011:369460. 10.1155/2011/369460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himi E., Nisar A., Noda K. (2005). Colour genes (R and Rc) for grain and coleoptile upregulate flavonoid biosynthesis genes in wheat. Genome 48, 747754. 10.1139/g05-026 [DOI] [PubMed] [Google Scholar]
- Himi E., Noda K. (2005). Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 143, 239–242. 10.1007/s10681-005-7854-4 [DOI] [Google Scholar]
- Himi E., Taketa S. (2015). Isolation of candidate genes for the barley Ant1 and wheat Rc genes controlling anthocyanin pigmentation in different vegetative tissues. Mol. Genet. Genom. 290, 1287–1298. 10.1007/s00438-015-0991-0 [DOI] [PubMed] [Google Scholar]
- Holtekjølen A. K., Sahlstrø S., Knutsen S. H. (2011). Phenolic contents and antioxidant activities in covered whole grain flours of Norwegian barley varieties and fractions obtained after pearling. Acta Agric. Scand. Sect. B Soil Plant Sci. 61, 67–74. 10.1080/09064710903496527 [DOI] [Google Scholar]
- Hu Y., Cheng Z., Heller L. I., Krasnoff S. B., Glahn R. P., Welch R. M. (2006). Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human Caco-2 cell model. J. Agric. Food Chem. 54, 9254–9261. 10.1021/jf0612981 [DOI] [PubMed] [Google Scholar]
- Hu Q.-P., Xu J.-G. (2011). Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 59, 2026–2033. 10.1021/jf104149q [DOI] [PubMed] [Google Scholar]
- Huang S.-H., Ng L.-T. (2012). Quantification of polyphenolic content and bioactive constituents of some commercial rice varieties in Taiwan. J. Food Comp. Anal. 26, 122–127. 10.1016/j.jfca.2012.03.009 [DOI] [Google Scholar]
- Huang D., Ou B., Hampsch-Woodill M., Flanagan J., Prior R. (2002). Using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem, 50, 4427–4444. 10.1021/jf0201529 [DOI] [PubMed] [Google Scholar]
- Ignat I., Volf I., Popa V. (2011). A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 126, 1821–1835. 10.1016/j.foodchem.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Jaafar S. N. S., Baron J., Siebenhandl-Ehn S., Rosenau T., Böhmdorfer S., Grausgruber H. (2013). Increased anthocyanin content in purple × blue aleurone wheat crosses. Plant Breed. 132, 546–552. 10.1111/pbr.12090 [DOI] [Google Scholar]
- Janik L. J., Cozzolino D., Dambergs R., Cynkar W., Gishen M. (2007). The prediction of total anthocyanin concentration in red grape homogenates using visible-near-infrared spectroscopy and artificial neural networks. Anal. Chim. Acta 594, 107–118. 10.1016/j.aca.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Jin L., Xiao P., Lu Y., Shao Y., Shen Y., Bao J. (2009). Quantitative trait loci for brown rice color, phenolics, flavonoid content, and antioxidant capacity in rice grain. Cereal Chem. 86, 609–615. 10.1094/CCHEM-86-6-0609 [DOI] [Google Scholar]
- Juzoń K., Skrzypek E., Czyczlo-Mysza I., Marcińska I. (2013). Effect of soil drought on the yield structure, protein and phenolics content in Pisum sativum and Lupinus luteus. Acta Agronom. Hungarica 61, 267–278. 10.1556/AAgr.61.2013.4.3 [DOI] [Google Scholar]
- Kaur-Sawhney R., Tiburcio A. F., Altabella T., Galston A. W. (2003). Polyamines in plants: an overview. J. Cell Mol. Biol. 2, 1–12. [Google Scholar]
- Keppenne V. D., Baenziger S. (1990). Inheritance of the blue aleurone trait in diverse wheat crosses. Genome 33, 525–529. 10.1139/g90-078 [DOI] [Google Scholar]
- Kezhu T., Yuhua C., Weixian S., Xiaoda C. (2014). Detection of isoflavones content in soybean based on Hyperspectral Imaging Technology. Sensors Transducers 169, 55–60. [Google Scholar]
- Khaopha S., Senawong T., Jogloy S., Patanothai A. (2012). Comparison of total phenolic content and composition of individual phenolic acids in testae and testae-removed kernels of 15 Valencia-type peanut (Arachis hypogaea L.) genotypes. Afr. J. Biotechnol. 11, 15923–15930. 10.5897/AJB12.1389 [DOI] [Google Scholar]
- Khlestkina E. K. (2013). Genes determining coloration of different organs in wheat. Russ. J. Genet. Appl. Res. 3, 54–65. 10.1134/S2079059713010085 [DOI] [Google Scholar]
- Khlestkina E. K., Röder M. S., Börner A. (2010). Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.). Euphytica 171, 65–69. 10.1007/s10681-009-9994-4 [DOI] [Google Scholar]
- Khlestkina E. K., Shoeva O. Y., Gordeeva E. I. (2015). Flavonoid biosynthesis genes in wheat. Russ. J. Genet. Appl. Res. 5, 268–278. 10.1134/S2079059715030077 [DOI] [Google Scholar]
- Khoddami A., Wilkes M. A., Roberts T. H. (2013). Techniques for analysis of plant phenolic compounds. Molecules 18, 2328–2375. 10.3390/molecules18022328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-J., Hyun J.-N., Kim J.-A., Park J.-C., Kim M.-Y., Kim J.-G., et al. (2007). Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 55, 4802–4809. 10.1021/jf0701943 [DOI] [PubMed] [Google Scholar]
- Kim E. H., Kim S. L., Kim S. H., Chung I. M. (2012a). Comparison of isoflavones and anthocyanins in soybean [Glycine max (L.) Merrill] seeds of different planting dates. J. Agric. Food Chem. 60, 10196–10202. 10.1021/jf3031259 [DOI] [PubMed] [Google Scholar]
- Kim J.-K., Kim E.-H., Lee O.-K., Park S.-Y., Lee B., Kim S.-H., et al. (2013). Variation and correlation analysis of phenolic compounds in mung bean (Vigna radiata L.) varieties. Food Chem. 141, 2988–2997. 10.1016/j.foodchem.2013.05.060 [DOI] [PubMed] [Google Scholar]
- Kim J.-K., Kim E.-H., Park I., Yu B.-R., Lim J. D., Lee Y.-S., et al. (2014). Isoflavones profiling of soybean [Glycine max (L.) Merrill] germplasm and their correlations with metabolic pathways. Food Chem. 153, 258–264. 10.1016/j.foodchem.2013.12.066 [DOI] [PubMed] [Google Scholar]
- Kim E.-H., Ro H.-M., Kim S.-L., Kim H.-S., Chung I. M. (2012b). Analysis of isoflavones, phenolic, soyasapogenol, and tocopherol compounds in soybean [Glycine max (L.) Merrill] germplasms of different seed weights and origins. J. Agric. Food Chem. 60, 6045–6055. 10.1021/jf300463f [DOI] [PubMed] [Google Scholar]
- Kim E. H., Seguin P., Lee J. E., Yoon C. G., Song H. K., Ahn J. K., et al. (2011). Elevated ultraviolet-B radiation reduces concentrations of isoflavones and phenolic compounds in soybean seeds. J. Agron. Crop Sci. 197, 75–80. 10.1111/j.1439-037X.2010.00444.x [DOI] [Google Scholar]
- Kuhnen S., Lemos P. M. M., Campestrini L. H., Ogliari J. B., Dias P. F., Maraschin M. (2011). Carotenoid and anthocyanin contents of grains of Brazilian maize landraces. J. Sci. Food Agric. 91, 1548–1553. 10.1002/jsfa.4346 [DOI] [PubMed] [Google Scholar]
- Kumar J., Ablett G. R. (2010). The soybean improvement in North America, in All About Soybeans in Japanese, ed Kitamura K. (Tokyo: Science Forum; ), 24–33. [Google Scholar]
- Kumar V., Rani A., Rawal R., Mourya V., Putrevu J., Jhawar J., et al. (2015). Genetic diversity of soybean genotypes differing in isoflavones content as revealed by HPLC and SSR markers. Australian J. Crop Sci. 9, 844–852. [Google Scholar]
- Lago C., Cassani E., Zanzi C., Landoni M., Trovato R., Pilu R. (2014). Development and study of a maize cultivar rich in anthocyanins: colored polenta, a new functional food. Plant Breed. 133, 210–217. 10.1111/pbr.12153 [DOI] [Google Scholar]
- Lago C., Landoni M., Cassani C., Doria E., Nielsen E., Pilu R. (2013). Study and characterization of a novel functional food: purple corn. Mol. Breed. 31, 575–585. 10.1007/s11032-012-9816-6 [DOI] [Google Scholar]
- Lähteenmäki L. (2013). Claiming health in food products. Food Qual. Pref. 27, 196–201. 10.1016/j.foodqual.2012.03.006 [DOI] [Google Scholar]
- Laino P., Shelton D., Finnie C., De Leonardis A. M., Mastrangelo A. M., Svensson B., et al. (2010). Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics 10, 2359–2368. 10.1002/pmic.200900803 [DOI] [PubMed] [Google Scholar]
- Lampila P., Van Lieshout M., Gremmen B., Lähteenmäki L. (2009). Consumer attitudes towards enhanced flavonoid content in fruit. Food Res. Int. 42, 122–129. 10.1016/j.foodres.2008.09.002 [DOI] [Google Scholar]
- Laus M. N., Benedetto N. A., Caporizzi R., Tozzi D., Soccio M., Giuzio L., et al. (2015). Evaluation of phenolic antioxidant activity in grains of modern and old durum wheat genotypes by the Novel QUENCHERABTS approach. Plant Foods Hum. Nutr. 70, 207–214. 10.1007/s11130-015-0483-8 [DOI] [PubMed] [Google Scholar]
- Lee J. H. (2010). Identification and quantification of anthocyanins from the grains of black rice (Oryza sativa L.) varieties. Food Sci. Biotechnol. 19, 391–397. 10.1007/s10068-010-0055-5 [DOI] [Google Scholar]
- Li Q., He F., Zhu B., Liu B., Sun R., Duan C., et al. (2014). Comparison of distinct transcriptional expression patterns of flavonoid biosynthesis in Cabernet Sauvignon grapes from east and west China. Plant Physiol. Bioch. 84, 45–56. 10.1016/j.plaphy.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Li Y., Ma D., Sun D., Wang C., Zhang J., Xie Y., et al. (2015). Total phenolics, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. The Crop J. 3, 328–334. 10.1016/j.cj.2015.04.004 [DOI] [Google Scholar]
- Li L., Shewry P. R., Ward J. L. (2008). Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 56, 9732–9739. 10.1021/jf801069s [DOI] [PubMed] [Google Scholar]
- Lee S. J., Yan W., Ahn J. K., Chung I. M. (2003). Effects of year, site, genotype and their interactions on various soybean isoflavones. Field Crops Res. 81, 181–192. 10.1016/S0378-4290(02)00220-4 [DOI] [Google Scholar]
- Lin-Wang K., Bolitho K., Grafton K., Kortstee A., Karunairetnam A., McGhie T. K., et al. (2010). An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 10:50. 10.1186/1471-2229-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Liu Y., Pu Z., Wang J., Zheng Y., Li Y., et al. (2013). Regulation, evolution, and functionality of flavonoids in cereal crops. Biotechnol. Letts. 35, 1765–1780. 10.1007/s10529-013-1277-4 [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez L., Oliart-Ros R. M., Valerio-Alfaro G., Lee C.-H., Parkin K. L., Garcia H. S. (2009). Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol. 42, 1187–1192. 10.1016/j.lwt.2008.10.010 [DOI] [Google Scholar]
- Lozovaya V. V., Lygin A. V., Ulanov A. V., Nelson R. L., Daide J., Widholm J. M. (2005). Effect of temperature and soil moisture status during seed development on soybean seed isoflavone concentration and composition. Crop Sci. 45, 1934–1940. 10.2135/cropsci2004.0567 [DOI] [Google Scholar]
- Lu X., Rasco B. A. (2012). Determination of antioxidant content and antioxidant activity in foods using infrared spectroscopy and chemometrics: a review. Crit. Rev. Food Sci. Nutr. 52, 853–875. 10.1080/10408398.2010.511322 [DOI] [PubMed] [Google Scholar]
- Ludwig S. R., Habera L. F., Dellaporta S. L., Wessler S. R. (1989). Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcription activators and contain the myc-homology region. Proc. Natl. Acad. Sci. U.S.A. 86, 7092–7096. 10.1073/pnas.86.18.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukow O., Suchy J., Adams K., Brown D., Depauw R., Fox S., et al. (2012). Effect of solar radiation, plant maturity and post-harvest treatment on the color and phenolic and carotenoid contents in seed of red and white Canadian wheat. J. Plant Cell Sci. 3, 1–13. [Google Scholar]
- Luthria D. L., Pastor-Corrales M. A. (2006). Phenolic acid content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Comp. Anal. 19, 205–211. 10.1016/j.jfca.2005.09.003 [DOI] [Google Scholar]
- Maeda H., Yamaguchi T., Omoteno M., Takarada T., Fujita K., Murata K., et al. (2014). Genetic dissection of black grain rice by the development of a near isogenic line. Breed. Sci. 64, 134–141. 10.1270/jsbbs.64.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S., Das A., Sardana H. K. (2015). Image acquisition techniques for assessment of legume quality. Trends Food Sci. Tech. 42, 116–133. 10.1016/j.tifs.2015.01.001 [DOI] [Google Scholar]
- Mahan A. L., Murray S. C., Rooney L. W., Crosby K. M. (2013). Combining ability for total phenols and secondary traits in a diverse set of colored (red, blue, and purple) maize. Crop Sci. 53, 1248–1255. 10.2135/cropsci2012.06.0385 [DOI] [Google Scholar]
- Mapope N., Dakora F. D. (2013). Role of flavonoid and isoflavonoid molecules in symbiotic functioning and host-plant defense in the leguminosae, in The Chemistry for Sustainable Development in Africa, eds Gurib-Fakim A., Eloff J. N. (Berlin; Heidelberg: Springer; ), 33–48. [Google Scholar]
- Mahmoudi T., Oveisi M. R., Jannat B., Behzad M., Hajimahmoodi M., Sadeghi N. (2015). Antioxidant activity of Iranian barley grain cultivars and their malts. Afr. J. Food Sci. 9, 534–539. 10.5897/AJFS2014.1210 [DOI] [Google Scholar]
- Martinek P., Jirsa O., Veculová K., Chrpova J., Watanabe N., Burešová V., et al. (2014). Use of wheat gene resources with different grain colour in breeding. 64 Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs 2013, 75–78. [Google Scholar]
- Martini D., Taddei F., Ciccoritti R., Pasquini M., Nicoletti I., Corradini D., et al. (2015). Variation of total antioxidant activity and of phenolic acid, total phenolics and yellow coloured pigments in durum wheat (Triticum turgidum L. var. durum) as a function of genotype, crop year and growing area. J. Cereal Sci. 65, 175–185. 10.1016/j.jcs.2015.06.012 [DOI] [Google Scholar]
- Matsui K., Umemura Y., Ohme-Takagi M. (2008). AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 55, 954–967. 10.1111/j.1365-313X.2008.03565.x [DOI] [PubMed] [Google Scholar]
- Matus J. T., Poupin M. J., Canon P., Bordeu E., Alcalde J. A., Arce-Johnson P. (2010). Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol. Biol. 72, 607–620. 10.1007/s11103-010-9597-4 [DOI] [PubMed] [Google Scholar]
- Mazur W. M., Duke J. A., Wähälä K., Rasku S., Adlecreutz H. (1998). Isoflavonoids and lignans in legumes: nutritional and health aspects in humans. J. Nutr. Biochem. 9, 193–200. 10.1016/S0955-2863(97)00184-8 [DOI] [Google Scholar]
- McKeehen J. D., Busch R. H., Fulcher R. G. (1999). Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J. Agric. Food Chem. 47, 1476–1482. 10.1021/jf980896f [DOI] [PubMed] [Google Scholar]
- Meksem K., Njiti V. N., Banz W. J., Iqbal M. J., Kassem M. M., Hyten D. L., et al. (2001). Genomic regions that underlie soybean seed isoflavone content. J. Biomed. Biotechnol. 1, 38–44. 10.1155/S1110724301000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Díaz S., del Carmen Ortiz-Valerio M., Castaño-Tostado E., de Dios Figueroa-Cárdennas J., Reynoso-Camacho R., Ramos-Gómez M., et al. (2012). Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented Creole maize races (Zea mays L.). Plant Foods Hum. Nutr. 67, 442–449. 10.1007/s11130-012-0326-9 [DOI] [PubMed] [Google Scholar]
- Meng F. L., Han Y. P., Teng W. L., Li Y. G., Li W. B. (2011). QTL underlying resistance to soybean aphid (Aphis glycines Matsumura) through isoflavone-mediated antibiosis in soybean cultivar Zhongdou 27. Theor. Appl. Genet. 123, 1459–1465. 10.1007/s00122-011-1680-y [DOI] [PubMed] [Google Scholar]
- Mink P. J., Scraffold C. G., Barraj L. M., Harnack L., Hong C. P., Nettleton J. A., et al. (2007). Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am. J. Clin. Nutr. 85, 895–909. [DOI] [PubMed] [Google Scholar]
- Mishra J., Singh R. D., Jadoon V. S., Gusain M. P., Aradhana (2012). HPTLC profile of quercetin content of common bean (Uttarakhand) landraces growing in Uttarakhand. Am. J. Food Technol. 7, 96–100. 10.3923/ajft.2012.96.100 [DOI] [Google Scholar]
- Mladenka P., Macakova K., Filipsky T., Zatloukalova L., Jahodar L., Bovicelli P., et al. (2011). In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 105, 693–701. 10.1016/j.jinorgbio.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Mojica L., Meyer A., Berhow M. A., Gonzalez de Mejía E. (2015). Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α - amylase and α -glucosidase while diverse phenolic composition and concentration. Food Res. Int. 69, 38–48. 10.1016/j.foodres.2014.12.007 [DOI] [Google Scholar]
- Montilla E. C., Hillebrand S., Antezana A., Winterhalter P. (2011). Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 59, 7068–7074. 10.1021/jf201061x [DOI] [PubMed] [Google Scholar]
- Morris G. P., Rhodes D. H., Brenton Z., Ramu P., Thayil S. D., Hash C. T., et al. (2013). Dissecting genome-wide association signals for loss-of-function phenotypes in sorghum flavonoid pigmentation traits. G3 3, 2085–2094. 10.1534/g3.113.008417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpofu A., Sapirstein H. D., Beta T. (2006). Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 54, 1265–1270. 10.1021/jf052683d [DOI] [PubMed] [Google Scholar]
- Murphy S. E., Lee E. A., Woodrow L., Seguin P., Kumar J., Rajcan I., et al. (2009a). Genotype × Environment interaction for isoflavone content in soybean. Crop Sci. 49, 1313–1321. 10.2135/cropsci2008.09.0533 [DOI] [Google Scholar]
- Murphy S. E., Lee E. A., Woodrow L., Seguin P., Kumar J., Rajcan I., et al. (2009b). Association of seed and agronomic traits with isoflavone levels in soybean. Canadian J. Plant Sci. 89, 477–484. 10.4141/CJPS08148 [DOI] [Google Scholar]
- Narwal S., Thakur V., Sheoran S., Dahiya S., Jaswal S., Gupta R. K. (2014). Antioxidant activity and phenolic content of the Indian wheat varieties. J. Plant Biochem. Biotechnol. 23, 11–17. 10.1007/s13562-012-0179-1 [DOI] [Google Scholar]
- Ndakidemi P. A., Dakora F. D. (2003). Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Funct. Plant Biol. 30, 729–745. 10.1071/FP03042 [DOI] [PubMed] [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study. Lancet 384, 766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. L., Hammerschmidt R. (1992). Phenolic compounds and their role in disease resistance. Ann. Rev. Phytopathol. 30, 369–389. 10.1146/annurev.py.30.090192.002101 [DOI] [Google Scholar]
- Nikolova M., Dimitrova M., Tasheva K., Todorova R., Dimitrova M., Ravishankar G., et al. (2014). Comparison of antiradical activity and total phenolic content in seeds of five soybean cultivars by applying different extraction solvents. Genet. Plant Physiol. 4, 110–116. [Google Scholar]
- Nocella G., Kennedy O. (2012). Food health claims: what consumers understand. Food Policy 37, 571–580. 10.1016/j.foodpol.2012.06.001 [DOI] [Google Scholar]
- Noubissié J. B. T., Youmbi E., Njintang N. Y., Abatchoua M. A., Nguimboou R. M., Bell J. M. (2012). Inheritance of phenolic contents and antioxidant capacity of dehulled seeds in cowpea (Vigna unguiculata L. Walp.). Int. J. Agr. Agric. Res. 2, 7–18. [Google Scholar]
- Nyström L., Lampi A.-M., Andersson A. A. M., Kamal-Eldin A., Gebruers K., Courtin C. M., et al. (2008). Phytochemicals and dietary fiber components in rye varieties in the health grain diversity screens. J. Agric. Food Chem. 56, 9758–9766. 10.1021/jf801065r [DOI] [PubMed] [Google Scholar]
- Oikawa T., Maeda H., Oguchi T., Yamaguchi T., Tanabe N., Ebana K., et al. (2015). The birth of a black rice gene and its local spread by introgression. Plant Cell 27, 2401–2014. 10.1105/tpc.15.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojwang L. O., Dykes L., Awika J. M. (2012). Ultra-performance of liquid chromatography tandem quadrupole mass spectrometry profiling of anthocyanins and flavonols in cowpea (Vigna unguiculata) of varying genotypes. J. Agric. Food Chem. 60, 3735–3744. 10.1021/jf2052948 [DOI] [PubMed] [Google Scholar]
- Oomah B. D., Corbè A., Balasubramanian P. (2010). Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food Chem. 58, 8225–8230. 10.1021/jf1011193 [DOI] [PubMed] [Google Scholar]
- Osborne B. G. (2006). Applications of near infrared spectroscopy in quality screening of early-generation materials in cereal breeding programmes. J. Near Infrared Spec. 14, 93–101. 10.1255/jnirs.595 [DOI] [Google Scholar]
- Ovando-Martínez M., Guzmán-Maldonado S. H., Simsek S., Bello-Pérez L. A., Osorio-Díaz P. (2014). Effect of water regimes on dietary fiber, polyphenols and antioxidant capacity of black and pinto beans. Agric. Sci. 5, 342–352 10.4236/as.2014.54036 [DOI] [Google Scholar]
- Pandey A., Misra P., Bhambhani S., Bhatia C., Trivedi P. K. (2014). Expression of Arabidopsis MYB transcription factor, AtMYB111, in tobacco requires light to modulate flavonol content. Sci. Rep. 4:5018. 10.1038/srep05018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-Y., Hua S.-H., Lim S.-H., Jung J. Y., Lee S. M., Yeo Y., et al. (2012). Determination of phenolic acids in Korean rice (Oryza sativa L.) cultivars using Gas Chromatography-Time-of-Flight Mass. Food Sci. Biotechnol. 21, 1141–1148. 10.1007/s10068-012-0149-3 [DOI] [Google Scholar]
- Pattanaik S., Kong Q., Zaitlin D., Werkman J. R., Xie C. H., Patra B., et al. (2010). Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta 231, 1061–1076. 10.1007/s00425-010-1108-y [DOI] [PubMed] [Google Scholar]
- Peiffer D. S., Wang L., Zimmerman N. P., Carmella S. G., Kuo C., Chen J., et al. (2016). Dietary consumption of black raspberries or their anthocyanin constituents alters innate immune cell trafficking in esophageal cancer. Cancer Immunol. Res. 4, 72–82. 10.1158/2326-6066.CIR-15-0091 [DOI] [PubMed] [Google Scholar]
- Pereira D. M., Valentão P., Pereira J. A., Andrade P. B. (2009). Phenolics: from chemistry to biology. Molecules 14, 2202–2211. 10.3390/molecules14062202 [DOI] [Google Scholar]
- Perron N. R., Brumaghim J. L. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 53, 75–100. 10.1007/s12013-009-9043-x [DOI] [PubMed] [Google Scholar]
- Phommalath S., Teraishi M., Yoshikawa T., Saito H., Tsukiyama T., Nakazaki T., et al. (2014). Wide genetic variation in phenolic compound content of seed coats among black soybean cultivars. Breed. Sci. 64, 409–415. 10.1270/jsbbs.64.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta P. G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042. 10.1021/np9904509 [DOI] [PubMed] [Google Scholar]
- Pissard A., Pierna J. A. F., Baeten V., Sinnaeve G., Lognay G., Mouteau A., et al. (2013). Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy. J. Sci. Food Agric. 93, 238–244. 10.1002/jsfa.5779 [DOI] [PubMed] [Google Scholar]
- Porter L. J., Hrstich L. N., Chan B. G. (1986). The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25, 223–230. 10.1016/S0031-9422(00)94533-3 [DOI] [Google Scholar]
- Price M. L., Van Scoyoc S., Butler L. G. (1978). A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 26, 1214–1218. 10.1021/jf60219a031 [DOI] [Google Scholar]
- Primomo V. S., Poysa V., Ablett G. R., Jackson C. J., Gijzen M., Rajcan I. (2005). Mapping QTL for individual and total isoflavone content in soybean seeds. Crop Sci. 45, 2454–2462. 10.2135/cropsci2004.0672 [DOI] [Google Scholar]
- Qi X., Shuai Q., Chen H., Fan L., Zeng Q., He N. (2014). Cloning and expression analysis of anthocyanin biosynthetic genes in mulberry plants. Mol. Genet. Genom. 289, 783–793. 10.1007/s00438-014-0851-3 [DOI] [PubMed] [Google Scholar]
- Qiu Y., Liu Q., Beta T. (2009). Antioxidant activity of commercial wild rice and identification of flavonoid compounds in active fractions. J. Agric. Food Chem. 57, 7543–7551. 10.1021/jf901074b [DOI] [PubMed] [Google Scholar]
- Qualset C. O., Soliman K. M., Jan C. C., Dvorak J., Mcguire P. E., Vogt H. E. (2005). Registration of UC 66049 Triticum aestivum blue aleurone genetic stock. Crop Sci. 45, 432 10.2135/cropsci2005.0432 [DOI] [Google Scholar]
- Ragaee S., Guzar I., Abdel-Aal E.-S. M., Seetharaman K. (2012). Bioactive components and antioxidant capacity of Ontario hard and soft wheat varieties. Can. J. Plant Sci. 92, 9–30. 10.4141/cjps2011-100 [DOI] [Google Scholar]
- Rahman M. M., Lee K. E., Lee E. S., Matin M. N., Lee D. S., Yun J. S., et al. (2013). The genetic constitution of complementary genes Pp and Pb determine the purple color variation in pericarps with cyanidin-3-O-glucoside depositions in black rice. J. Plant Biol. 56, 24–31. 10.1007/s12374-012-0043-9 [DOI] [Google Scholar]
- Ramírez-Jiménez A. K., Reynoso-Camacho R., Tejero M. E., León-Galván F., Loarca- Piña G. (2015). Potential role of bioactive compounds of Phaseolus vulgaris L. on lipid-lowering mechanisms. Food Res. Int. 76, 92–104. 10.1016/j.foodres.2015.01.002 [DOI] [Google Scholar]
- Rascio A., Picchi V., Naldi J. P., Colecchia S., De Santis G., Gallo A., et al. (2015). Effects of temperature increase, through spring sowing, on antioxidant power and health-beneficial substances of old and new wheat. J. Cereal Sci. 61, 11–118. 10.1016/j.jcs.2014.09.010 [DOI] [Google Scholar]
- Razzaghi-As N., Garrido J., Khazraei H., Borges F., Firuzi F. (2013). Antioxidant properties of hydroxycinnamic acids: a review of structure-activity relationships. Curr. Med. Chem. 20, 123–135. [DOI] [PubMed] [Google Scholar]
- Rhodes D. H., Hoffmann L., Jr., Rooney W. L., Ramu P., Morris G. P., Kresovich S. (2014). Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J. Agric. Food Chem. 62, 10916–10927. 10.1021/jf503651t [DOI] [PubMed] [Google Scholar]
- Roberfroid M. B. (2002). Global view on functional foods: European perspectives. Br. J. Nutr. 88, S133–S138. 10.1079/bjn2002677 [DOI] [PubMed] [Google Scholar]
- Romani A., Vignolini P., Galardi C., Mulinacci N., Benedettelli S., Heimler D. (2004). Germplasm characterization of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content. J. Agric. Food Chem. 52, 3838–3842. 10.1021/jf0307402 [DOI] [PubMed] [Google Scholar]
- Rooney W. L., Portillo O., Hayes C. (2013a). Registration of ATx3363 and BTx3363 black sorghum germplasm. J. Plant Reg. 7, 342–346. 10.3198/jpr2013.01.0006crg [DOI] [Google Scholar]
- Rooney W. L., Rooney L. W., Awika J., Dykes L. (2013b). Registration of Tx3362 sorghum germplasm. J. Plant Reg. 7, 104–107. 10.3198/jpr2012.04.0262crg [DOI] [Google Scholar]
- Rosales-Serna R., Gutiérrez-Uribe J. A., Reyes-Barraza E., Mayek-Pérez N., Saldivar-Serna S. R. O. (2015). Genetic relationship among common bean cultivars with enhanced accumulation of bioactive compounds. J. Agric. Sci. 7, 106–116. 10.5539/jas.v7n9p106 [DOI] [Google Scholar]
- Rubiales D., Fondevilla S., Chen W., Gentzbittel L., Higgins T. J. V., Castillejo M. A., et al. (2015). Achievements and challenges in legume breeding for pest and disease resistance. Crit. Rev. Plant Sci. 34, 195–236. 10.1080/07352689.2014.898445 [DOI] [Google Scholar]
- Ryu S. N., Park S. Z., Ho C.-T. (1998). High performance liquid chromatographic determination of anthocyanin pigments in some varieties of black rice. J. Food Drug Anal. 6, 729–736. [Google Scholar]
- Saba A., Vassallo M., Shepherd R., Lampila P., Arvola A., Dean M., et al. (2010). Country-wise differences in perception of health-related messages in cereal-based food products. Food Qual. Pref. 21, 385–393. 10.1016/j.foodqual.2009.09.007 [DOI] [Google Scholar]
- Santiago R., Barros-Rios J., Malvar R. A. (2013). Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 14, 6960–6980. 10.3390/ijms14046960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer-Lequart C., Lehmann U., Ross A. B., Roger O., Eldridge A. L., Ananta E., et al. (2015). Whole grain in manufactured foods: current use, challenges and the way forward. Crit. Rev. Food Sci. Nutr. [Epub ahead of print]. 10.1080/10408398.2013.781012. [DOI] [PubMed] [Google Scholar]
- Schaich K. M., Tian X., Xie J. (2015). Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DDPH, and ORAC assays. J. Funct. Foods 14, 111–125. 10.1016/j.jff.2015.01.043 [DOI] [Google Scholar]
- Schmutz J., Cannon S. B., Schlueter J., Ma J., Mitros T., Nelson W., et al. (2010). Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183. 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- Schwinn K., Venail J., Shang Y., Mackay S., Alm V., Butelli E., et al. (2006). A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 18, 831–851. 10.1105/tpc.105.039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev A., Badani H., Kapulnik Y., Shomer I., Oren-Shamir M., Galili S. (2010). Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.). J. Food Sci. 75, S115–S119. 10.1111/j.1750-3841.2009.01477.x [DOI] [PubMed] [Google Scholar]
- Shao Y., Tang F., Huang Y., Xu F., Chen Y., Tong C., et al. (2014a). Analysis of Genotype × Environment interactions for polyphenols and antioxidant capacity of rice by association mapping. J. Agric. Food Chem. 62, 5361–5368. 10.1021/jf500951e [DOI] [PubMed] [Google Scholar]
- Shao Y., Xu F., Sun X., Bao J., Beta T. (2014b). Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 143, 90–96. 10.1016/j.foodchem.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Sharma M., Chai C., Morohashi K., Grotewold E., Snook M. E., Chopra S. (2012). Expression of flavonoid 3′-hydroxylase is controlled by P1, the regulator of 3-deoxyflavonoid biosynthesis in maize. BMC Plant Biol. 12:196. 10.1186/1471-2229-12-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Cortes-Cruz M., Ahern K. R., McMullen M., Brutnell T. P., Chopra S. (2011). Identification of the Pr1 gene product completes the anthocyanin biosynthesis pathway of maize. Genetics 188, 69–79. 10.1534/genetics.110.126136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Kaur R., Mangat G. S., Singh K. (2014). Red pericarp introgression lines derived from interspecific crosses of rice: physiochemical characteristics, antioxidative properties and phenolic content. J. Sci. Food Agric. 94, 2912–2920. 10.1002/jsfa.6632 [DOI] [PubMed] [Google Scholar]
- Sharma S., Thakur D. R. (2014). Biochemical basis for bruchid resistance in cowpea, chickpea and soybean genotypes. Am. J. Food Technol. 9, 318–324. 10.3923/ajft.2014.318.324 [DOI] [Google Scholar]
- Shen Y., Jin L., Xiao P., Lu Y., Bao J. (2009). Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 49, 106–111. 10.1016/j.jcs.2008.07.010 [DOI] [Google Scholar]
- Shen Y., Shen J., Dawadondup Zhuang, L., Wang Y., Pu J., et al. (2013). Physical localization of a novel blue-grained gene derived from Thinopyrum bessarabicum. Mol. Breed. 31, 195–204. 10.1007/s11032-012-9783-y [DOI] [Google Scholar]
- Shewry P. R. (2009). The HEALTHGRAIN programme opens new opportunities for improving wheat for nutrition and health. Nutr. Bull. 34, 225–231. 10.1111/j.1467-3010.2009.01747.x [DOI] [Google Scholar]
- Shewry P. R., Hey S. (2015). Do ancient wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 65, 236–243. 10.1016/j.jcs.2015.07.014 [DOI] [Google Scholar]
- Shoeva O. Y., Gordeeva E. L., Khlestkina E. K. (2014). The regulation of anthocyanin synthesis in the wheat pericarp. Molecules 19, 20266–20279. 10.3390/molecules191220266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenhandl-Ehn S., Grausgruber H., Pellegrini N., Del Rio D., Fogliano V., Pernice R., et al. (2007). Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J. Agric. Food Chem. 55, 8541–8547. 10.1021/jf072021j [DOI] [PubMed] [Google Scholar]
- Siebenhandl-Ehn S., Kinner M., Leopold L. F., Poppernitsch M. B., Prückler M., Wurbs P., et al. (2011). Hulless Barley—A Rediscovered source for Functional Foods Phytochemical Profile and Soluble Dietary Fiber Content in Naked Barley Varieties and their Antioxidant Properties. Available online at: http://cdn.intechopen.com/pdfs-wm/25502.pdf
- Singleton V. L., Orthofer R., Lamuela-Raventos R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 299, 152−178. 10.1016/S0076-6879(99)99017-1 [DOI] [Google Scholar]
- Siro I., Kapolna E., Kapolna B., Lugasi A. (2008). Functional food. Product development, marketing and consumer acceptance: a review. Appetite 51, 456–467. 10.1016/j.appet.2008.05.060 [DOI] [PubMed] [Google Scholar]
- Soares S., Kohl S., Thalmann S., Mateus N., Meyerhof W., de Freitas V. (2013). Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 61, 1525–1533. 10.1021/jf304198k [DOI] [PubMed] [Google Scholar]
- Sompong R., Siebenhandl-Ehn S., Linsberger-Martin G., Berghofer E. (2011). Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China, and Sri Lanka. Food Chem. 124, 132–140. 10.1016/j.foodchem.2010.05.115 [DOI] [Google Scholar]
- Spelt C., Quattrocchio F., Mol J. N., Koes R. (2000). Anthocyanin1 of petunia encodes a basic helix–loop–helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 12, 1619–1632. 10.1105/tpc.12.9.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriseadka T., Wongpornchai S., Rayanakorn M. (2012). Quantification of flavonoids in black rice by liquid chromatography-negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 60, 11723–11732. 10.1021/jf303204s [DOI] [PubMed] [Google Scholar]
- Stracke R., Ishihara H., Huep G., Barsch A., Mehrtens F., Niehaus K., et al. (2007). Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50, 660–677. 10.1111/j.1365-313X.2007.03078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf B., Yan F., Honermeier B. (2015). Nitrogen fertilization and maturity influence the phenolic concentration of wheat grain (Triticum aestivum). J. Plant Nutr. Soil Sci. 178, 118–125. 10.1002/jpln.201400139 [DOI] [Google Scholar]
- Subramanian S., Stacey G., Yu O. (2007). Distinct, crucial roles of flavonoids during legume nodulation. Trends Plant Sci. 12, 82–85. 10.1016/j.tplants.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Svensson L., Sekwati-Monang B., Lutz D. L., Schieber A., Gänzle M. G. (2010). Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 58, 9214–9220. 10.1021/jf101504v [DOI] [PubMed] [Google Scholar]
- Sweeney M. T., Thomsoon M. J., Pfeil B. E., McCouch S. (2006). Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 18, 283–294. 10.1105/tpc.105.038430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tako E., Glahn R. P. (2010). White beans provide more bioavailable iron than red beans: studies in poultry (Gallus gallus) and an in vitro digestion/Caco-2 model. Int. J. Vitam. Nutr. Res. 80, 416–429. 10.1024/0300-9831/a000028 [DOI] [PubMed] [Google Scholar]
- Tako E., Reed S. M., Budiman J., Hart J. J., Glahn R. P. (2015). Higher iron pearl millet (Pennisetum glaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutr. J. 14, 1–11. 10.1186/1475-2891-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleon V., Dykes L., Rooney W. L., Rooney L. W. (2012). Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J. Cereal Chem. 56, 470–475. 10.1016/j.jcs.2012.05.001 [DOI] [Google Scholar]
- Taleon V., Dykes L., Rooney W. L., Rooney L. W. (2014). Environmental effect on flavonoid concentrations and profiles of red and lemon-yellow sorghum grains. J. Food Compos. Anal. 34, 178–185. 10.1016/j.jfca.2014.03.003 [DOI] [Google Scholar]
- Tereshchenko O. Y., Gordeeva E. I., Arbuzova V. S., Börner A., Khlestkina E. K. (2012). The D genome carries a gene determining purple grain colour in wheat. Cereal Res. Commun. 40, 334–341. 10.1556/CRC.40.2012.3.2 [DOI] [Google Scholar]
- Teucher B., Olivares M., Cori H. (2004). Enhancers of iron absorption: ascorbic acid and other organic acids. Int. J. Vit. Nutr. Res. 74, 403–419. 10.1024/0300-9831.74.6.403 [DOI] [PubMed] [Google Scholar]
- Tian X., Schaich K. M. (2013). Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS. J. Agric. Food Chem. 61, 5511–5519. 10.1021/jf4010725 [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Quail P. H. (2003). The Arabidopsis basic/ helix–loop–helix transcription factor family. Plant Cell 15, 1749–1770. 10.1105/tpc.013839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. (2015). Global cancer statistics. CA-Cancer J. Clin. 65, 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- Treutte D. (2006). Significance of flavonoids in plant resistance: a review. Environ. Chem. Letts. 4, 147–157. 10.1007/s10311-006-0068-8 [DOI] [Google Scholar]
- Upadhyaya H. D., Ortiz R. (2001). A mini core subset for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement. Theor. Appl. Genet. 102, 1292–1298. 10.1007/s00122-001-0556-y [DOI] [Google Scholar]
- Urias-Lugo D. A., Heredia J. B., Serna-Saldivar S. O., Muy-Rangel M. D., Valdez-Torres J. B. (2015). Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea mays l.). CyTA J. Food 13, 336–339. 10.1080/19476337.2014.980324 [DOI] [PubMed] [Google Scholar]
- Urias-Peraldí M., Gutiérrez-Uribe J. A., Perciado-Ortiz R. E., Cruz-Morales A. S., Serna-Saldívar S. O., García-Lara S. (2013). Nutraceutical profiles improved blue maize (Zea mays L.) hybrids for subtropical regions. Field Crops Res. 141, 69–76. 10.1016/j.fcr.2012.11.008 [DOI] [Google Scholar]
- Van der Kamp J. W. (2012). Paving the way for innovation in enhancing the intake of whole grain. Trends Food Sci. Technol. 25, 101–107. 10.1016/j.tifs.2011.12.005 [DOI] [Google Scholar]
- Verma B., Hucl P., Chibbar R. N. (2008). Phenolic content and antioxidant properties of grain in 51 wheat cultivars. Cereal Chem. 85, 544–549. 10.1094/CCHEM-85-4-0544 [DOI] [Google Scholar]
- Wagner K.-H., Brath H. (2012). A global view on the development of non communicable diseases. Prev. Med. 54, S38–S48. 10.1016/j.ypmed.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Wang Y., Frei M. (2011). Stressed food - The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 141, 271–286. 10.1016/j.agee.2011.03.017 [DOI] [Google Scholar]
- Wang M. L., Gillaspie A. G., Morris J. B., Davis J., Pederson G. A. (2008). Flavonoid content in different legume germplasm seeds quantified by HPLC. Plant Genet. Resour. 6, 62–69. 10.1017/S1479262108923807 [DOI] [Google Scholar]
- Wang Y., Han Y., Teng W., Zhao X., Li Y., Wu L., et al. (2014). Expression quantitative trait loci infer the regulation of isoflavone accumulation in soybean (Glycine max L. Merr.) seed. BMC Genom. 15:680. 10.1186/1471-2164-15-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Han Y., Zhao X., Li Y., Teng W., Li D., et al. (2015). Mapping isoflavone QTL with main, epistatic and QTL × environment effects in recombinant inbred lines of soybean. PLoS ONE 10:e0118447. 10.1371/journal.pone.0118447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. L., Morris J. B. (2007). Flavonoid content in seeds of guar germplasm using HPLC. Plant Genet. Resour. 5, 96–99. 10.1017/S1479262107672335 [DOI] [Google Scholar]
- Wang C., Sherrard M., Pagadala S., Wixon R., Scott R. A. (2000). Isoflavone content among maturity group 0 to II soybeans. J. Am. Oil Chem. Soc. 77, 483–487. 10.1007/s11746-000-0077-6 [DOI] [Google Scholar]
- Wang C., Shu Q. (2007). Fine mapping and candidate gene analysis of purple pericarp gene Pb in rice (Oryza sativa L.). Chin. Sci. Bull. 52, 3097–3104. 10.1007/s11434-007-0472-x [DOI] [Google Scholar]
- Waniska R. D., Rooney L. W. (2000). Structure and chemistry of the sorghum caryopsis, in The Sorghum: Origin, History, Technology, and Production, eds Smith C. W., Frederiksen R. A. (New York, NY: John Wiley and Sons, In,), 649–687. [Google Scholar]
- Ward J. L., Poutanen K., Gebruers K., Piironsen V., Lampi A.-M., Nystrőm L., et al. (2008). The HEALTHGRAIN cereal diversity screen: concept, results, and prospects. J. Agric. Food Chem. 56, 9699–9709. 10.1021/jf8009574 [DOI] [PubMed] [Google Scholar]
- Warner E. F., Zhang Q., Raheem K. S., O'Hagan D., O'Connell M. A., Kay C. D. (2016). Common phenolic metabolites of flavonoids, but not their un-metabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 146, 465–473. 10.3945/jn.115.217943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson J. J., Butler L. G. (1983). Occurrence of an unusual leucoanthocyanidin and absence of proanthocyanidins in sorghum leaves. J. Agric. Food Chem. 31, 41–45. 10.1021/jf00115a011 [DOI] [Google Scholar]
- Wei H., Zhu J.-H., Shang Y., Jia Q.-J., Wang J.-M., Yang J.-M. (2013). Research advanced in colored barley. J. Plant Genet. Resour. 14, 1020–1024. [Google Scholar]
- Weseler A. R., Ruijters E. J., Drittij-Reijnders M. J., Reesink K. D., Haenen G. R., Bast A. (2011). Pleiotropic benefit of monomeric and oligomeric flavanols on vascular health – a randomized controlled clinical study. PLoS ONE 6:e28460. 10.1371/journal.pone.0028460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre E., Oliver J., van den Broek R., van Engelen P., Kremers B., van der Horst B., et al. (2003). Predictive analysis of cocoa procyanidins using near-infrared spectroscopy techniques. J. Food Sci. 68, 2618–2622. 10.1111/j.1365-2621.2003.tb05779.x [DOI] [Google Scholar]
- Wickert E., Schiocchet M. A., Noldin J. A., Raimondi J. A., de Andrade A., Scheuermann K. K., et al. (2014). Exploring variability: New Brazilian varieties SCS119 Rubi and SCS120 Onix for the specialty rices market. Open J. Genet. 4, 157–165. 10.4236/ojgen.2014.42016 [DOI] [Google Scholar]
- Wilson R. F. (ed.). (2012). Designing Soybeans for 21st Century Markets. Urbana, IL: AOCS Press. [Google Scholar]
- Wu J. G., Shi C. H. (2004). Prediction of grain weight, brown rice weight and amylose content in single rice grains using near-infrared reflectance spectroscopy. Field Crop Res. 87, 13–21. 10.1016/j.fcr.2003.09.005 [DOI] [Google Scholar]
- Wu J. G., Shi C. H. (2007). Calibration model optimization for rice cooking characteristics by near infrared reflectance spectroscopy (NIRS). Food Chem. 103, 1054–1061. 10.1016/j.foodchem.2006.07.063 [DOI] [Google Scholar]
- Wu J. G., Shi C. H., Zhang X. M. (2002). Estimating the amino acid composition in the milled rice powder by near-infrared reflectance spectroscopy. Field Crop Res. 75, 1–7. 10.1016/S0378-4290(02)00006-0 [DOI] [Google Scholar]
- Xie J., Schaich K. M. (2014). Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 62, 4251–4260. 10.1021/jf500180u [DOI] [PubMed] [Google Scholar]
- Yang L., Allred C. D., Awika J. M. (2014). Emerging evidence on the role of estrogenic sorghum flavonoids in colon cancer prevention. Cereal Foods World 59, 244–251. 10.1094/CFW-59-5-0244 [DOI] [Google Scholar]
- Yang R.-Y., Lin S., Kuo G. (2008). Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 17, 275–279. [PubMed] [Google Scholar]
- Yang K., Moon J. K., Jeong N., Chun H. K., Kang S. T., Back K., et al. (2011). Novel major quantitative trait loci regulating the content of isoflavone in soybean seeds. Genes Genom. 33, 685–692. 10.1007/s13258-011-0043-z [DOI] [Google Scholar]
- Yoshimura Y., Zaima N., Moriyama T., Kawamura Y. (2012). Different localization patterns of anthocyanin species in the pericarp of black rice revealed by imaging mass spectrometry. PLoS ONE 7:e31285. 10.1371/journal.pone.0031285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G., Li D., Han Y., Teng W., Wang J., Qiu L., et al. (2009). Identification of QTL underlying isoflavone contents in soybean seeds among multiple environments. Theor. Appl. Genet. 118, 1455–1463. 10.1007/s00122-009-0994-5 [DOI] [PubMed] [Google Scholar]
- Zeven A. C. (1991). Wheats with purple and blue grains: a review. Euphytica 56, 243–258. 10.1007/BF00042371 [DOI] [Google Scholar]
- Zhang J., Ge Y., Han F., Li B., Yan S., Sun J., et al. (2014). Isoflavone content of soybean cultivars from maturity group 0 to VI grown in northern and southern China. J. Am. Oil Chem. Soc. 91, 1019–1028. 10.1007/s11746-014-2440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shao Y., Bao J., Beta T. (2015). Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 172, 630–639. 10.1016/j.foodchem.2014.09.118 [DOI] [PubMed] [Google Scholar]
- Zhang C., Shen Y., Chen J., Xiao P., Bao J. (2008). Nondestructive prediction of total phenolics, flavonoid contents, and antioxidant capacity of rice grain using near-infrared spectroscopy. J. Agric. Food Chem. 56, 8268–8272. 10.1021/jf801830z [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang J., Shen J., Silva A., Dennis D. A., Barrow C. J. (2006). A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 18, 445–450. 10.1007/s10811-006-9048-4 [DOI] [Google Scholar]
- Zhang M. W., Zhang R. F., Zhang F. X., Liu R. H. (2010). Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 58, 7580–7587. 10.1021/jf1007665 [DOI] [PubMed] [Google Scholar]
- Zhang R. F., Zhang F. X., Zhang M. W., Zhen C. W., Yang C. Y., Zhang Y., et al. (2011). Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 59, 5935–5944. 10.1021/jf201593n [DOI] [PubMed] [Google Scholar]
- Zheng Q., Li B., Li H., Li Z. (2009). Utilization of blue-grained character in wheat breeding derived from Thinopyrum poticum. J. Genet. Genom. 36, 575–580. 10.1016/S1673-8527(08)60149-6 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Li B., Mu S., Zhou H., Li Z. (2006). Physical mapping of the blue-grained gene(s) from Thinopyrum ponticum by GISH and FISH in a set of translocation lines with different seed colors in wheat. Genome 49, 1109–1114. 10.1139/g06-073 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Robards K., Helliwell S., Blanchard C. (2004). The distribution of phenolic acids in rice. Food Chem. 87, 401–406. 10.1016/j.foodchem.2003.12.015 [DOI] [Google Scholar]
- Zhu F., Cai Y. Z., Bao J., Corke H. (2010). Effect of γ-irradiation on phenolic compounds in rice grain. Food Chem. 120, 74–77. 10.1016/j.foodchem.2009.09.072 [DOI] [Google Scholar]
- Zhu H. F., Fitzsimmons K., Khandelwal A., Kranz R. G. (2009). CPC, a single-repeat R3MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis. Mol. Plant 2, 790–802. 10.1093/mp/ssp030 [DOI] [PubMed] [Google Scholar]
- Žilić S., Serpen A., Akıllıoğlu G., Gökmen V., Vančetović J. (2012). Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 60, 1224–1231. 10.1021/jf204367z [DOI] [PubMed] [Google Scholar]
- Žofajova A., Pšenáková I., Havrlentová M., Piliarová M. (2012). Accumulation of total anthocyanins in wheat grain. Agriculture 58, 50–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.