Abstract
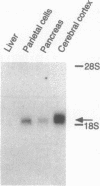
Gastrin is an important stimulant of acid secretion by gastric parietal cells and is structurally related to the peptide hormone cholecystokinin (CCK). The pharmacologic properties of the parietal cell gastrin receptor are very similar to the predominant CCK receptor in the brain, CCK-B. Neither the gastrin nor the CCK-B receptor have been cloned thus far, making it difficult to resolve whether these two receptors are distinct. We have isolated a clone encoding the canine gastrin receptor by screening a parietal cell cDNA expression library using a radioligand-binding strategy. Nucleotide sequence analysis revealed an open reading frame encoding a 453-amino acid protein with seven putative hydrophobic transmembrane domains and significant homology with members of the beta-adrenergic family of G protein-coupled receptors. The expressed recombinant receptor shows the same binding specificity for gastrin/CCK agonists and antagonists as the canine parietal cell receptor. Gastrin-stimulated phosphatidylinositol hydrolysis and intracellular Ca2+ mobilization in COS-7 cells expressing the cloned receptor suggest second-messenger signaling through phospholipase C. Affinity labeling of the expressed receptor in COS-7 cells revealed a protein identical in size to the native parietal cell receptor. Gastrin receptor transcripts were identified by high-stringency RNA blot analysis in both parietal cells and cerebral cortex, suggesting that the gastrin and CCK-B receptors are either highly homologous or identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Chandler R., Scanlon D. B., Weinstock J. Identification of a gastrin binding protein in porcine gastric mucosal membranes by covalent cross-linking with iodinated gastrin. J Biol Chem. 1986 Sep 15;261(26):12252–12257. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew C. S., Brown M. R. Release of intracellular Ca2+ and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta. 1986 Aug 29;888(1):116–125. doi: 10.1016/0167-4889(86)90077-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Eva C., Keinänen K., Monyer H., Seeburg P., Sprengel R. Molecular cloning of a novel G protein-coupled receptor that may belong to the neuropeptide receptor family. FEBS Lett. 1990 Oct 1;271(1-2):81–84. doi: 10.1016/0014-5793(90)80377-u. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fourmy D., Zahidi A., Fabre R., Guidet M., Pradayrol L., Ribet A. Receptors for cholecystokinin and gastrin peptides display specific binding properties and are structurally different in guinea-pig and dog pancreas. Eur J Biochem. 1987 Jun 15;165(3):683–692. doi: 10.1111/j.1432-1033.1987.tb11495.x. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klueppelberg U. G., Gaisano H. Y., Powers S. P., Miller L. J. Use of a nitrotryptophan-containing peptide for photoaffinity labeling the pancreatic cholecystokinin receptor. Biochemistry. 1989 Apr 18;28(8):3463–3468. doi: 10.1021/bi00434a047. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Kaji E. H., Winkel G. K., Ives H. E., Lodish H. F. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Park J., Yamada T. Gastrin receptor characterization: affinity cross-linking of the gastrin receptor on canine gastric parietal cells. Am J Physiol. 1987 Jan;252(1 Pt 1):G143–G147. doi: 10.1152/ajpgi.1987.252.1.G143. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Muallem S., Sachs G. Changes in cytosolic free Ca2+ in isolated parietal cells. Differential effects of secretagogues. Biochim Biophys Acta. 1984 Oct 12;805(2):181–185. doi: 10.1016/0167-4889(84)90166-6. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. K., Powers S. P., Hadac E. M., Gaisano H., Miller L. J. Establishment of a new short, protease-resistant, affinity labeling reagent for the cholecystokinin receptor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):346–353. doi: 10.1016/s0006-291x(87)80128-6. [DOI] [PubMed] [Google Scholar]
- Rajan A. S., Hill R. S., Boyd A. E., 3rd Effect of rise in cAMP levels on Ca2+ influx through voltage-dependent Ca2+ channels in HIT cells. Second-messenger synarchy in beta-cells. Diabetes. 1989 Jul;38(7):874–880. doi: 10.2337/diab.38.7.874. [DOI] [PubMed] [Google Scholar]
- Roche S., Bali J. P., Galleyrand J. C., Magous R. Characterization of a gastrin-type receptor on rabbit gastric parietal cells using L365,260 and L364,718. Am J Physiol. 1991 Feb;260(2 Pt 1):G182–G188. doi: 10.1152/ajpgi.1991.260.2.G182. [DOI] [PubMed] [Google Scholar]
- Roche S., Bali J. P., Magous R. Involvement of a pertussis toxin-sensitive G protein in the action of gastrin on gastric parietal cells. Biochim Biophys Acta. 1990 Dec 10;1055(3):287–294. doi: 10.1016/0167-4889(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Roche S., Gusdinar T., Bali J. P., Magous R. Biphasic kinetics of inositol 1,4,5-trisphosphate accumulation in gastrin-stimulated parietal cells. Effects of pertussis toxin and extracellular calcium. FEBS Lett. 1991 Apr 22;282(1):147–151. doi: 10.1016/0014-5793(91)80465-f. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]