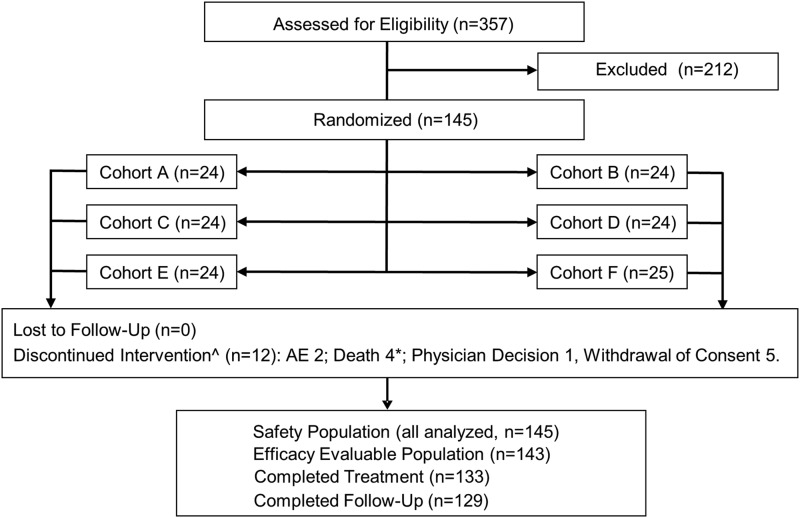
Figure 1.
Patient disposition. AE, adverse event. *One patient died during the follow-up period. ^Four of the 12 discontinuations of treatment occurred in cohorts A and B (16 weeks of treatment) after 1.1, 4.7, 13.3, and 13.4 weeks of dosing. The remaining eight discontinuations occurred in cohorts C–F (24 weeks of treatment) after 1 day (single dose) and 2.6, 3.4, 8.1, 8.9, 11.1, 18.1, and 21.7 weeks of dosing.