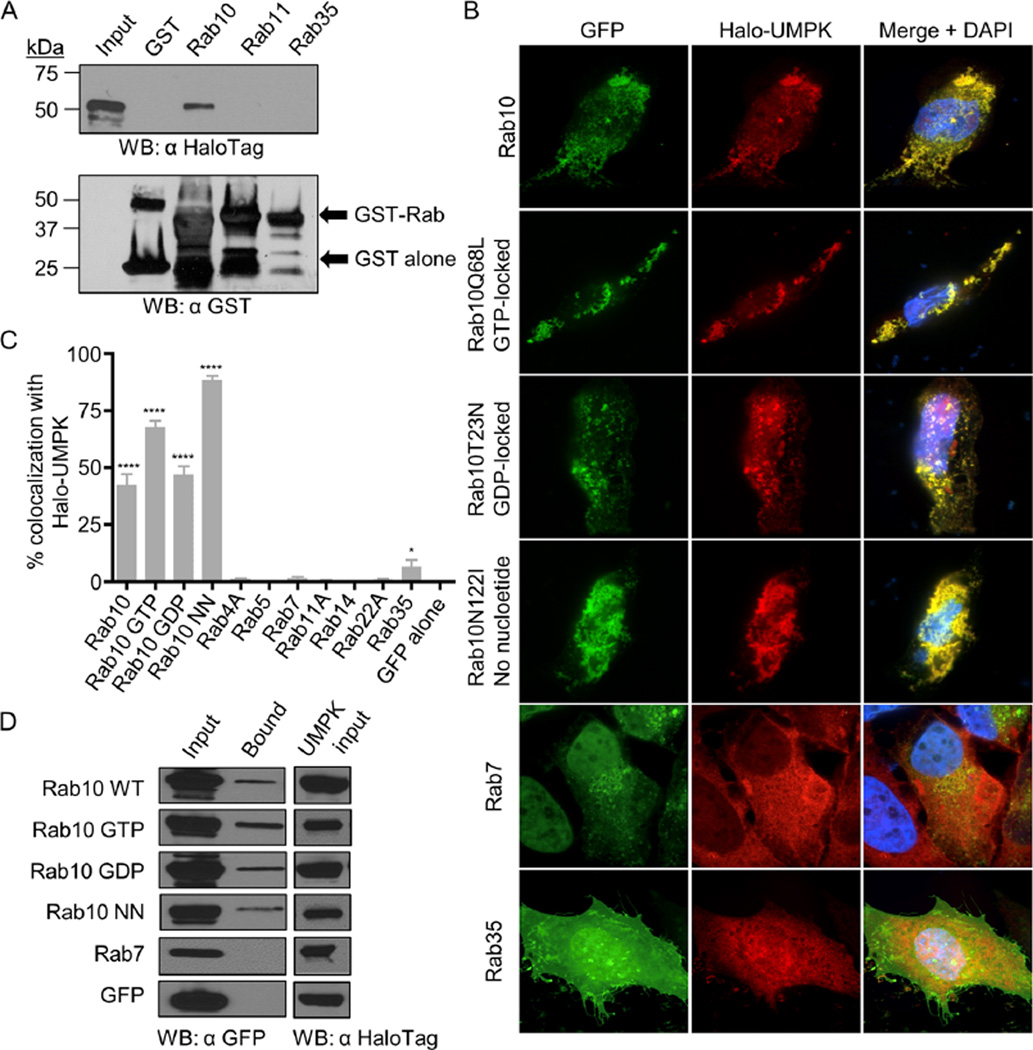
Fig. 10.
Validation of A. phagocytophilum UMPK-Rab10 interactions.
A. GST-Rab10 but not other GST-tagged Rabs or GST alone pull down UMPK. A lysate of E. coli expressing Halo-UMPK was divided equally and incubated with GST, GST-Rab10, GST-Rab11 and GST-Rab35 coated beads. Input E. coli lysate and bead eluates were analysed by immunoblot using HaloTag and GST antibodies.
B. Halo-UMPK colocalizes specifically with vesicles that are positive for GFP-Rab10 isoforms in an aggregative pattern. HeLa cells were cotransfected to express Halo-UMPK together with GFP-Rab10 isoforms or other GFP-tagged Rab GTPases and analysed by confocal microscopy. Rab10Q68L (GTP locked); Rab10T32N (GDP locked); Rab10N122I [incapable of binding any GTP or GDP (no nucleotide)].
C. Vesicles that are positive for GFP-Rab10 isoforms differentially colocalize with UMPK. One hundred cells coexpressing Halo-tagged A. phagocytophilum UMPK and GFP-tagged Rab GTPases or Halo-tagged E. coli UMPK and GFP-Rab10 were assessed per condition for Halo and GFP signal colocalization. Statistically significant (****P ≤ 0.0001; *P ≤ 0.05) values are indicated.
D. Halo-UMPK interacts with all Rab10 isoforms in living cells. HeLa cells were cotransfected to express Halo-UMPK together with GFP-Rab10 isoforms, GFP-Rab7 or GFP alone. Whole-cell lysates of the transfected cells (Input) were mixed with Halo-Link resin, and the resin eluate (Bound) was analysed by Western blot (WB) with GFP antibody. Portions of each transfection lysate were analysed with HaloTag antibody to confirm Halo-UMPK bait expression (UMPK input). Data presented in A and D are representative of two experiments with similar results. Data presented in B and C are representative of three experiments with similar results.