Systolic and diastolic function affect dilated cardiomyopathy (DCM) outcomes. However, systolic:diastolic (S:D) coupling, as a distinct characteristic, may itself affect function but is poorly characterized. Using tissue Doppler echocardiography, we found that S:D coupling becomes weaker in DCM with left ventricular remodeling and dysfunction and that the S:D coupling ratio may be useful to assess coupling, warranting study in relation to patient outcomes.
Keywords: systole, diastole, cardiomyopathy, pediatrics, echocardiography
Abstract
Systolic and diastolic function affect dilated cardiomyopathy (DCM) outcomes. However, systolic-diastolic coupling, as a distinct characteristic, may itself affect function but is poorly characterized. We hypothesized that echocardiographic left ventricular (LV) longitudinal systolic tissue velocities (S') correlate with diastolic longitudinal velocities (E') and that their relationship is associated with ventricular function and that this relationship is impaired in pediatric DCM. We analyzed data from the Pediatric Heart Network Ventricular Volume Variability study, using linear regression and generalized additive modeling to assess relationships between S' and E' at the lateral and septal mitral annulus. We explored relationships between the systolic:diastolic (S:D) coupling ratio (S':E' relative to age) and ventricular function. Up to 4 echocardiograms from 130 DCM patients (mean age: 9.3 ± 6.1 yr) and 1 echocardiogram from each of 591 healthy controls were analyzed. S' and E' were linearly related in controls (r = 0.64, P < 0.001) and DCM (r = 0.83, P < 0.001). In DCM, the magnitude of association between S' and E' was reduced with progressive ventricular remodeling. The S:D ratio was more strongly associated with LV function in controls vs. DCM. The septal S:D ratio was higher (presumed worse) in DCM vs. controls (0.69 ± 0.13 vs. 0.62 ± 0.12, P = 0.001). A higher septal S:D ratio was associated with worse LV dimensions (parameter estimate: 0.0061, P = 0.004), mass (parameter estimate: 0.0074, P = 0.002), ejection fraction (parameter estimate: −0.0303, P = 0.024), and inflow propagation (parameter estimate: −0.3538, P < .001). S:D coupling becomes weaker in DCM with LV remodeling and dysfunction. The S:D coupling ratio may be useful to assess coupling, warranting study in relation to patient outcomes.
NEW & NOTEWORTHY
Systolic and diastolic function affect dilated cardiomyopathy (DCM) outcomes. However, systolic:diastolic (S:D) coupling, as a distinct characteristic, may itself affect function but is poorly characterized. Using tissue Doppler echocardiography, we found that S:D coupling becomes weaker in DCM with left ventricular remodeling and dysfunction and that the S:D coupling ratio may be useful to assess coupling, warranting study in relation to patient outcomes.
both systolic and diastolic dysfunctions are present in dilated cardiomyopathy (DCM) and both contribute to morbidity and mortality in this condition (28). In clinical practice, systolic and diastolic functions are traditionally considered as two separate and distinct phases. However, the systolic and diastolic behaviors of the left ventricle (LV) are physiologically linked through several mechanisms. Diastolic influence on systole (diastolic → systolic coupling) is the most familiar and is mediated through the Frank-Starling and other mechanisms enhance systolic contraction. Systolic influence on diastole (systolic → diastolic coupling) is mediated through enhancement of early diastolic filling by myocardial elastic recoil in early diastole (24, 25).
Although in normal hearts reciprocal coupling between systole and diastole enhances cardiac performance (37), experimental data have yielded conflicting results on whether the Frank-Starling mechanism is disrupted in DCM (17, 34, 35). The profound impairment of ventricular molecular and myocardial systolic and diastolic properties that is characteristic of DCM may disrupt diastolic → systolic coupling through reduced preload reserve and impair systolic → diastolic coupling secondary to the diminished end-systolic deformation that is associated with reduced fiber shortening. Therefore, assessment of systolic and diastolic function, and of the coupling between them, the subject of this study, is of interest. Defining abnormal linking between systolic and diastolic function as a distinct entity may itself hold important information regarding patient cardiac status and potentially regarding clinical outcomes beyond evaluation of systolic and diastolic function alone. While several papers demonstrate that systolic and diastolic function are linked (24, 25, 37), systolic-diastolic (S:D) coupling as distinct functional entities has not been systematically evaluated in either adult or pediatric DCM, and although theoretically predicted, it remains unknown whether impaired coupling is associated with cardiac dysfunction. Moreover, S:D coupling is not routinely investigated or accounted for clinically and to date there is no accepted method for assessing S:D coupling in clinical practice. Gold standard methods for assessing systolic and diastolic function in this population include measures of contractility such as end-systolic elastance and ventricular filling pressures, respectively. However, these necessitate invasive catheterization and even then end-systolic elastance is not routinely measured. Echocardiography is commonly used to assess cardiac function in DCM and tissue Doppler imaging can provide measures of systolic and diastolic function in the same cardiac sample. Although twist-untwist is a key mechanism linking systolic and diastolic function (7), assessment of twist-untwist is relatively cumbersome by two-dimensional echocardiography. A more simple and practical approach, and presumably more feasible in clinical practice, is to study the longitudinal vector of the overall twist-untwist action of the LV, readily achievable using tissue Doppler echocardiography.
Using tissue Doppler echocardiography to assess systolic and diastolic longitudinal function and their coupling, we previously found that mitral annular systolic longitudinal velocities correlate linearly with diastolic velocities in healthy children and in those with hypertrophic or DCM (22). This relationship varied between the groups, implying that S:D coupling may be differentially affected by the specific disease (22). We also found that the ratio of peak systolic (S') to peak early diastolic (E') longitudinal myocardial velocities (=S'/E'), a putative index of S:D coupling, correlated with mitral inflow deceleration time (22). As there is no currently accepted index of S:D coupling used in clinical routine, a direct ratio between indexes of systolic and diastolic function may be useful. As correlations between systolic and diastolic velocities cannot be used to assess S:D coupling in the individual patient (as correlations cannot be derived from a single measurement in one individual), the S:D coupling ratio z-score (defined as the z-score relative to age of the ratio of peak S' divided by peak E') may be a potential clinically applicable parameter to indicate S:D coupling (22). This ratio is based on the following concept: if S:D coupling is reduced, and the energy built up in systole is not fully transferred into diastole, we would expect less diastolic response for a given systolic velocity and thus a higher S:D coupling ratio. However, our initial single-center study was limited by its relatively small size and its retrospective nature. The aim of the current exploratory study was to utilize a large, multicenter, prospectively collected, relatively homogenous cohort of children with DCM, analyzed in a core echocardiographic laboratory, to: 1) investigate the relation of systolic to diastolic tissue velocities as an indicator of S:D coupling; 2) investigate the relation of S:D coupling to parameters of ventricular size and function in pediatric DCM; and 3) compare these results with normal controls. We hypothesized that LV longitudinal systolic velocities are normally highly correlated with diastolic longitudinal velocities, that their relationship is related to ventricular size and function, and that S:D coupling is impaired in DCM compared with healthy controls.
METHODS
This study was a subanalysis of the Pediatric Heart Network Ventricular Volume Variability (VVV) database, which aimed to determine the interstudy variability of echocardiographically derived LV measurements in pediatric DCM patients and the variance of change in z-score on serial follow-up (5). The z-scores reflect the number of SDs from the parameter mean for a particular population or age group relative to the distribution in a normal population, thereby allowing comparison of values for children over the pediatric age range by adjusting for the effects of age and body size. The z-score for the mean of a normal population distribution is 0, and the normal range is typically defined as −2 to +2 SD. In brief, children or young adults age <22 yr with known or suspected DCM disease duration >2 mo, anticipated longitudinal follow-up to occur at the same institution, and informed consent were prospectively enrolled at eight centers between the years 2005 and 2007 (5). Echocardiographic eligibility criteria included LV dilation [end-diastolic dimension (EDD) >5.5 cm or z-score >2] and LV dysfunction [ejection fraction (EF) <50%, shortening fraction <28%, or z-score for either >−2]. Exclusion criteria included other forms of cardiomyopathy, noncompaction, congenital heart disease, nonsinus rhythm, and hemodynamic instability (5). Repeat echocardiographic assessment was performed on return visits up to 18 mo following enrollment. The mean time between the first and follow-up echocardiogram (performed closest to a 12-mo follow-up target) was 9.1 ± 3.5 (median 9.6, range 2 to 18) mo. In some patients a third, and in two cases a fourth, follow-up echocardiogram was available and included in the analysis.(5)
Normal Data
As the VVV study did not include a normal control cohort, we used z-scores derived from a large database of normal controls collected at a single center (Boston Children's Hospital) as a reference for the S:D coupling ratio and to explore associations between S:D coupling and ventricular function (3). The characteristics of this normal population and z-score methodologies have been previously described (3, 4). In brief, subjects were either normal volunteers or were referred for screening for conditions such as murmur, mitral valve prolapse, or bicuspid aortic valve. These subjects had normal echocardiograms, no history of disease, normal height, weight, and blood pressure for age. In normal controls, the echocardiogram was obtained at a single visit. Pulsed tissue velocities were prospectively acquired at the mitral lateral and septal annuli, as described for the DCM cohort. Peak S' and E' were measured in the same core laboratory that measured echo parameters for the VVV study. For the current analysis, we used existing measurements from the core laboratory database.
Echocardiography
Echocardiographic methodologies have been previously detailed and were performed according to recently published pediatric guidelines (5, 19). The current analysis used LV volumes and mass calculated using the (5/6) × area × length method and dimensions from two-dimensional echocardiography as the (5/6) × area × length method had better reproducibility than other methods in recent pediatric studies (19, 21). LV mass was expressed as a z-score. Wall stress was derived from the Laplace equation, provided by the ultrasound software, using the blood pressure, LV dimension, and wall thickness. Pulsed tissue Doppler (TDI) measurements were acquired from the apical four-chamber view at the mitral lateral and septal annulus and the peak systolic (S'), early diastolic (E'), and late diastolic (A') TDI velocities recorded. When TDI E' and A' waves were fused, the “summation” wave was taken as the peak diastolic wave. Peak velocities were averaged over three beats. For the current study, echocardiographic data from the primary image acquisition obtained at each time point, averaged across three beats using the core laboratory primary reader's interpretation from the VVV database, were used.
Assessing Relationships Between Systolic and Diastolic Velocities
Relationships between systolic and diastolic function were explored by assessing correlations between S' and E' at the lateral and septal mitral annulus.
S:D Coupling Ratio
Expressing the S:D coupling ratio as a z-score allowed for investigation of its association with parameters of ventricular size, remodeling, and function, which are related to age. The S:D coupling ratio was calculated at the lateral mitral annulus and the septal annulus, as the average of three successive beats.
Statistical Analysis
Descriptive statistics include the median with interquartile range for skewed variables, mean ± SD for other continuous variables, and frequency with percentage for categorical variables. Peak systolic and peak early diastolic velocity and S:D coupling index z-score were compared by t-test or Wilcoxon rank sum test as appropriate to assess differences between the two cohorts. The within-subject correlation coefficient (the proportion of variation in systolic velocity explained by diastolic velocity after removing the variation due to measurement on different subjects) was calculated to assess whether the increase in systolic velocity over time is associated with diastolic velocity within the same individual. Between-subject correlation was calculated by using a weighted average of the number of repeated measurements available for each subject (33). Linear regression with a random effect for subject and a compound symmetry covariance structure to account for correlation among repeated measures and a fixed continuous time effect denoting time since the baseline echo was used to model the serial measures of S:D coupling index to identify the impact of LV size and function on the S:D coupling index. Linear regression, piecewise linear regression, and generalized additive modeling (GAM) were used to assess the association between systolic and diastolic velocities. Tests of interaction were used to assess the impact of LV size and function on the strength of association between systolic and diastolic velocities. LV size and function measures (e.g., LV EDD z-score) were categorized using quartiles to report estimated slopes by magnitude of LV size and function. In testing associations, S' and E' were sometimes placed on the “dependent” (y-axis) and sometimes the “independent” variable (x-axis). In reality, systole and diastole are reciprocally linked both being dependent on one another.
Linear regression was also used to identify the impact of LV size and function on the S:D coupling index. As an absolute LV EDD >5.5 cm was an inclusion criterion (and may correspond to a z-score of <2 in some patients), and as regression analyses included data from all visits, with some patients improving LV dimensions over time, data with LV EDD z-score <2 are included. The number and type of significant findings from the two cohorts were compared to assess whether S:D coupling is weaker in DCM subjects relative to normal controls. All analyses were conducted using SAS version 9.2 (Statistical Analysis System, Cary, NC) and R version 2.13.0. The study was approved by the institutional review committees at each institution. Subjects gave informed consent.
RESULTS
The current study included 130 fully eligible patients for the VVV study, 130 of whom had baseline echocardiography, at a mean age of 9.3 ± 6.1 yr (5). Of these, 23 patients had echocardiography only at baseline, 70 had one follow-up echocardiogram at a mean interval of 7.1 ± 3.5 mo, 28 had 2 follow-up echocardiograms at a mean interval of 10.9 ± 3.1 mo, 7 had 3 follow-up echocardiograms at a mean interval of 11.3 ± 2.5 mo, and 2 had 4 follow-up echocardiograms. Patient characteristics are presented in Table 1. The majority of subjects had known or suspected idiopathic or familial DCM and were in New York Heart Association or Ross classification class 1 or 2. TDI velocities and the S:D ratio are summarized in Table 2. At baseline, diastolic summation waves were present in 19 (14.6%) patients.
Table 1.
Dilated cardiomyopathy subject characteristics at baseline
| Variable | n | |
|---|---|---|
| Age at echocardiography, yr | 130 | 9.3 ± 6.1 (median = 9.3) |
| Height, cm | 128.0 ± 36.7 | |
| Weight, kg | 37.7 ± 27.6 | |
| Gender | ||
| Male | 58 | 44.6% |
| Female | 72 | 55.4% |
| Primary causes of dilated cardiomyopathy | ||
| Idiopathic or familial | 118 | 90.7% |
| Metabolic disorder | 2 | 1.5% |
| Mitochondrial disorder | 2 | 1.5% |
| Neuromuscular disease | 4 | 3.1% |
| Single gene defect | 4 | 3.1% |
| Congestive heart failure classification | ||
| Ross classification (age <5 yr) | 45 | |
| Class I | 35 | 77.8% |
| Class II | 6 | 13.3% |
| Class III | 4 | 8.9% |
| Class IV | 0 | 0% |
| New York Heart classification (age ≥5 yr) | 85 | |
| Class I | 50 | 58.8% |
| Class II | 33 | 38.8% |
| Class III | 2 | 2.4% |
| Class IV | 0 | 0% |
| Medications at baseline echo | ||
| Antithrombotic | 36 | 28.0% |
| β-blocker | 68 | 52.0% |
| Angiotensin converting enzyme inhibitor | 109 | 84.0% |
| Diuretic | 68 | 52.0% |
| Glycoside | 77 | 59.0% |
Table 2.
Systolic and diastolic tissue Doppler velocities and their ratio at the lateral mitral and septal annulus
| Dilated Cardiomyopathy Baseline Echocardiogram (n = 130) |
Controls (n = 591) |
t-Test |
Wilcoxon |
|||||
|---|---|---|---|---|---|---|---|---|
| Raw value, cm/s | z-Score (means ± SD) | Median | Raw value, cm/s | z-Score (means ± SD) | Median | P value | P value | |
| Lateral mitral annulus | ||||||||
| Diastolic velocity (E') | 12.69 ± 4.59 | −1.60 ± 1.66 | −1.68 | 17.29 ± 4.18 | −0.02 ± 1.06 | −0.09 | <.0001 | <.0001 |
| Systolic velocity (S') | 6.44 ± 2.60 | −1.49 ± 1.43 | −1.75 | 9.65 ± 2.51 | 0.03 ± 0.98 | −0.04 | <.0001 | <.0001 |
| Septal annulus | ||||||||
| Diastolic velocity (E') | 8.48 ± 2.70 | −2.19 ± 1.35 | −2.01 | 12.74 ± 2.63 | 0.01 ± 0.96 | −0.04 | <.0001 | <.0001 |
| Systolic velocity (S') | 5.73 ± 1.77 | −1.79 ± 2.22 | −2.00 | 7.63 ± 1.27 | 0.12 ± 1.03 | 0.05 | <.0001 | <.0001 |
| S:D ratio index z-score | ||||||||
| Left lateral annulus | 0.58 ± 0.14 | −0.20 ± 1.33 | −0.55 | 0.54 ± 0.19 | 0.01 ± 1.02 | −0.15 | 0.128 | 0.001 |
| Septal annulus | 0.69 ± 0.13 | 0.87 ± 1.40 | 0.85 | 0.62 ± 0.12 | 0.00 ± 1.06 | −0.13 | <.0001 | <.0001 |
Normal controls included 591 subjects (283 females). Their demographics were as follows: age 10.38 ± 5.47 yr (range: 0.01- 20.50); 62 were <2 yr old; weight: 41.10 ± 22.66 kg (range: 1.23-99.70); height: 139.73 ± 33.96 cm (range: 46.00–196.00); and body surface area: 1.23 ± 0.51 cm2 (range: 0.11–2.30); all had normal body mass index: 19.04 ± 3.49 (range: 10.80–29.45).
Relationship Between Systolic and Diastolic Velocities in Normal Controls
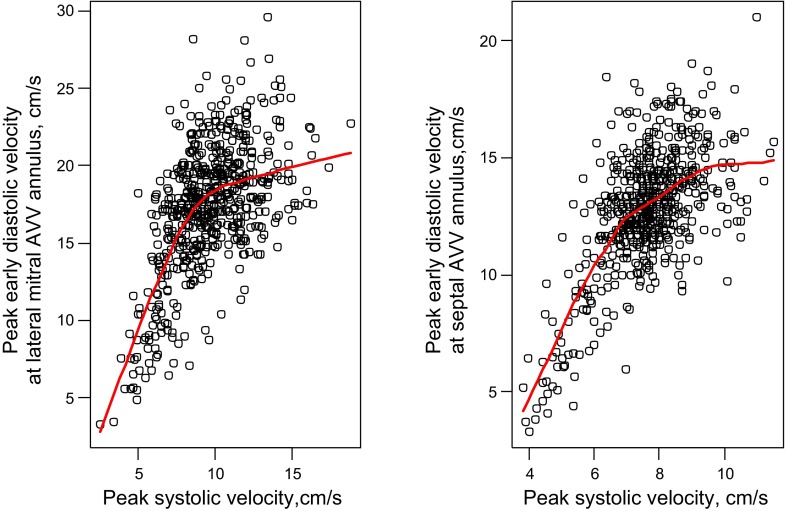
Diastolic and systolic velocities were positively correlated at the lateral mitral and septal annuli. A one-unit increase in systolic velocity was associated with a 0.96 unit increase in diastolic velocities at the lateral mitral annulus (r = 0.58; P < 0.001) and a 1.32 unit increase (r = 0.64, P < 0.001) in diastolic velocities at the septal annulus. However, at both locations, a nonlinear relationship between these variables was present (GAM P <0.001 for both) with little association at high velocities, i.e., ∼10 cm/s or higher (Fig. 1). Results were unchanged when controls younger than age 2 mo were excluded to have the normal cohort more closely resemble the DCM cohort.
Fig. 1.
Relationship between the tissue Doppler peak early diastolic velocity E' (y-axis) and the peak systolic velocity S' (x-axis) at the lateral mitral (top) and septal (bottom) annulus in normal controls. A 1-unit increase in systolic velocity was associated with a 0.96 unit increase in diastolic velocities at the lateral mitral annulus (r = 0.58; P < 0.001) and a 1.32 unit increase (r = 0.64, P < 0.001) in diastolic velocities at the septal annulus. Nonlinearity P < 0.001. AVV, atrioventricular valve.
Relationship Between Systolic and Diastolic Velocities in DCM Patients
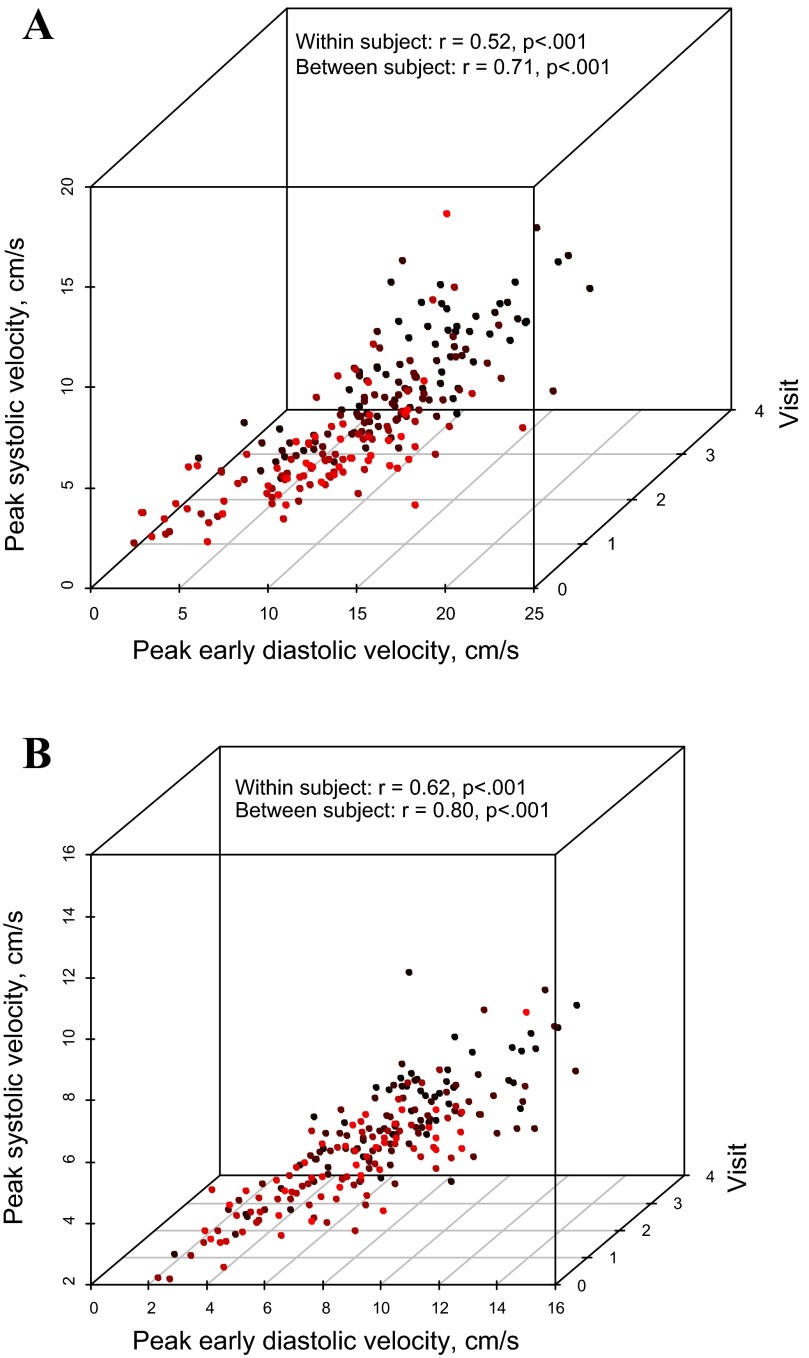
Relationships between systolic and diastolic velocities in the DCM cohort are presented in Fig. 2. Moderate correlations between systolic and diastolic tissue velocities were observed at the lateral mitral annulus (Fig. 2A). This relationship was relatively constant over the duration of follow-up. The relationship between systolic and diastolic velocities was highest at the septal annulus (r = 0.83, 0.76, 0.66, and 0.80 at baseline through third follow-up visit, respectively; P < 0.001 for all; Fig. 2B).
Fig. 2.
Relationship between the tissue Doppler peak early diastolic velocity E' (y-axis) and the peak systolic velocity S' (x-axis) at the lateral mitral (A) and septal (B) annulus in dilated cardiomyopathy (DCM) patients. These are positively correlated at baseline and at follow-up visits 1–3. The correlation coefficients account for the repeated measurements per subject. Data points from different study visits are displayed with different colors.
Within-subject correlation analysis showed that an increase in the lateral mitral annulus peak systolic velocity was associated with an increase in peak early diastolic velocity (r = 0.52, P < 0.001). Between-subject correlation coefficient analysis showed that higher S' values correlated with higher E' velocities (r = 0.71, P < 0.001). Similarly, within-subject Pearson's correlation for the septal annulus was r = 0.62 and between-subjects correlation was r = 0.80 (P < 0.001 for both). Nonlinear modeling did not reveal stronger associations between the two parameters.
Impact of LV Size and Function on Systolic-Diastolic Coupling
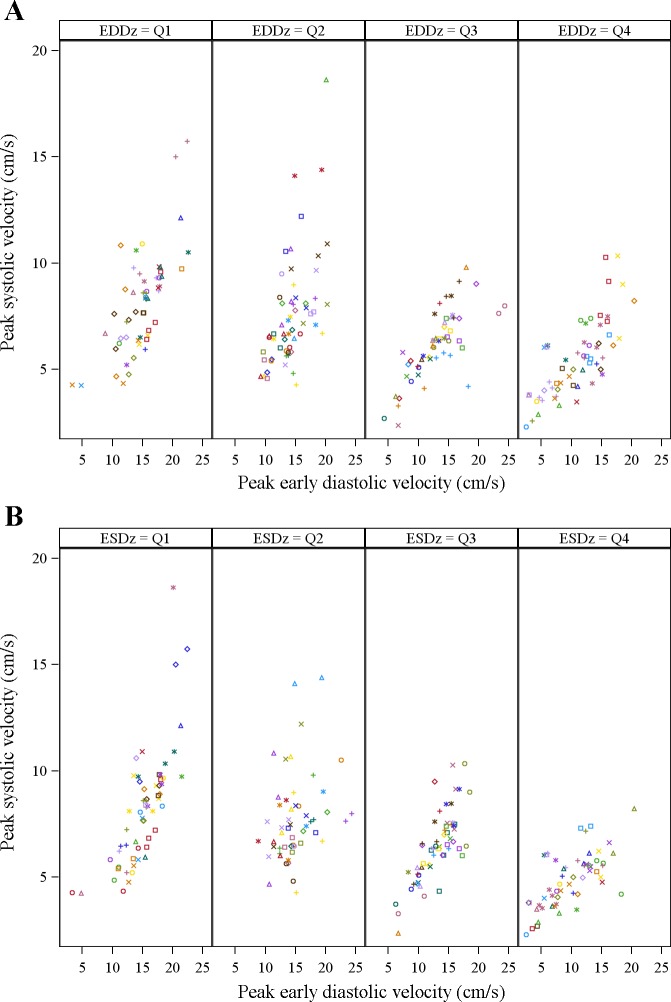
To examine whether LV size and function impact the association between longitudinal systolic-diastolic coupling, an interaction term was included in the mixed effects regression model. This term assesses if the association between diastolic and systolic velocities is stronger at higher or lower levels of ventricular function. We found that both EDD and end-systolic dimension (ESD) impacted the relationship between peak early systolic and diastolic velocities at the lateral mitral annulus (interaction P = 0.016 and 0.049, respectively). Patients with larger LV EDD had weaker correlations between systolic and diastolic velocities with the slope decreasing by 0.03 for every one-unit increase in peak early diastolic velocity (interaction P = 0.006; Fig. 3A). The slope estimates of peak S' vs. peak E' varied by size of the LV EDD z-score: a one-unit increase in E' was associated with a 0.37 unit increase in peak systolic velocity when the LV EDD z-score was 1.87 (25th percentile), 0.33 when LV EDD z-score was 3.19 (median), and 0.28 when the LV EDD z-score was 4.96 (75th percentile). Similarly, among patients with an average ESD z-score in the top quartile (>75th percentile), overall S:D coupling was weaker (but still positive) compared with patients with average LV ESD z-score in the lowest quartile (<25th percentile; Fig. 3B). There was no interaction with LV EF (P = 0.77) or with raw LV mass (P = 0.39) or mass-to-volume ratio (P = 0.11).
Fig. 3.
Relationships between the tissue Doppler peak early diastolic velocity E' (y-axis) and the peak systolic velocity S' (x-axis) at the lateral mitral annulus in DCM patients stratified by severity of left ventricular (LV) dilation in quartiles (Q1-4) of severity. Relationships stratified by end-diastolic dimension (EDD) z-score (A) and by end-systolic dimension (ESD) z-score (B). For end-diastolic dimension z-score the 25th; 50th and 75th percentiles are 1.9, 3.3, and 5.4, respectively. For end-systolic dimension z-score the 25th; 50th and 75th percentiles are 4.4, 6.4, and 9.9, respectively. Data points from different study visits are displayed with different colors. The slope of the S:D relationship decreases with increasing LV remodeling.
Effect of Myocardial Shortening and Wall Stress
We identified a significant interaction (P = 0.027) between E' and velocity of fiber shortening z-score at the lateral mitral annulus. The association between systolic and diastolic velocities strengthens as velocity of fiber shortening z-score increases (increase in slope for peak S' vs. E' of 0.011 per one-unit increase fiber shortening velocity z-score).
We also identified a significant interaction (P = 0.027) between E' and end-systolic fiber stress z-score. That is, the prediction of systolic velocity by diastolic velocity gets weaker as end-systolic fiber stress z-score increases (slope for peak S' vs. E' decreases by 0.02 per each one-unit increase in end-systolic fiber stress z-score). However, as the velocity of fiber shortening itself depends on end-systolic wall stress, which in turn is influenced by ESD, we examined the two significant interactions after adjusting for ESD z-score. After adjustment, the interaction between E' and fiber shortening velocity z-score becomes nonsignificant (P = 0.90). Similarly, after adjustment for ESD z-score, the interaction between E' and end-systolic fiber stress z-score also becomes nonsignificant (P = 0.99). Thus the dependence of coupling magnitudes for both velocity of shortening and end-systolic stress is influenced predominantly by underlying LV systolic size.
Systolic-Diastolic Relationships Expressed as a Systolic-Diastolic Coupling Ratio: Normal Controls
In healthy controls the S:D coupling ratio was (mean ± SD) 0.58 ± 0.14 at the mitral lateral annulus (n = 591) and 0.62 ± 0.12 at the septal annulus (n = 590).
Relationships between the S:D coupling ratio and functional parameters in normal controls.
MITRAL LATERAL ANNULUS.
The results from all tested associations are presented in the appendix (see Table A1). Linear associations between the mitral lateral S:D coupling ratio and other functional indexes with P < 0.1 in normal controls are summarized in Table 3. A higher S:D ratio z-score was associated with: older age at echocardiography, male gender, higher systolic blood pressure, larger ventricular dimensions and mass, smaller sphericity index, higher velocity of fiber shortening, and lower peak early diastolic velocity.
Table A1.
Univariate linear regressions of the S:D ratio raw value and z-score on patient and echocardiographic characteristics at the lateral mitral annulus among normal controls
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 591 | 0.0012 | 0.232 | 0.0456 | <.001 |
| Gender (male vs. female) | 591 | 0.0292 | 0.012 | 0.1784 | 0.034 |
| SBP-for-age z-score | 558 | 0.0214 | <.001 | 0.1276 | 0.003 |
| DBP-for-age z-score | 557 | 0.0121 | 0.035 | 0.0564 | 0.183 |
| ESD-for-BSA z-score | 531 | −0.0130 | 0.033 | −0.0933 | 0.036 |
| End diastolic volume, ml | 575 | 0.0001 | 0.243 | 0.0050 | <.001 |
| EDV-for-BSA z-score | 574 | −0.0185 | 0.002 | −0.0970 | 0.025 |
| End systolic volume, ml | 574 | 0.0003 | 0.325 | 0.0117 | <.001 |
| ESV-for-BSA z-score | 574 | −0.0314 | <.001 | −0.2409 | <.001 |
| Ventricular mass, g | 575 | 0.0003 | 0.038 | 0.0063 | <.001 |
| Mass-for-BSA z-score | 574 | −0.0066 | 0.267 | −0.0323 | 0.456 |
| Mass-to-volume ratio | 575 | 0.1743 | <.001 | 0.9910 | 0.002 |
| MV-for-age z-score | 575 | 0.0215 | <.001 | 0.1389 | <.001 |
| Sphericity index | 523 | −0.3563 | <.001 | −3.0216 | <.001 |
| Sphericity-for-age z-score | 523 | −0.0229 | <.001 | −0.1792 | <.001 |
| Shortening fraction, % [2D] | 531 | 0.0019 | 0.312 | −0.0048 | 0.729 |
| Shortening fraction-for-age z-score | 531 | −0.0017 | 0.801 | −0.0052 | 0.915 |
| Velocity of fiber shortening, circ/s | 476 | 0.2335 | <.001 | 1.0542 | 0.013 |
| VCFc-for-age z-score | 476 | 0.0171 | 0.005 | 0.1240 | 0.005 |
| Ejection fraction, % | 575 | −0.0458 | 0.724 | −0.1971 | 0.834 |
| EF-for-age z-score | 575 | −0.0006 | 0.915 | 0.0041 | 0.924 |
| End-systolic meridional fiber stress, g/cm2 | 481 | −0.0004 | 0.384 | 0.0041 | 0.198 |
| End-systolic circumferential fiber stress, g/cm2 | 477 | −0.0002 | 0.463 | 0.0025 | 0.145 |
| Peak early velocity, m/s | 459 | −0.2194 | <.001 | −1.2407 | <.001 |
| Peak early velocity-for-age z-score | 459 | −0.0345 | <.001 | −0.2487 | <.001 |
| Peak atrial velocity, m/s | 422 | 0.1371 | 0.008 | 0.5649 | 0.139 |
| Peak A-wave velocity-for-age z-score | 420 | 0.0076 | 0.166 | 0.0397 | 0.327 |
| Early deceleration time, ms | 419 | 0.0000 | 0.964 | 0.0023 | 0.063 |
| Deceleration time-for-age z-score | 419 | −0.0049 | 0.461 | −0.0435 | 0.370 |
| Left ventricular flow propagation velocity, cm/s | 224 | −0.0001 | 0.756 | 0.0001 | 0.964 |
| LV flow propagation-for-age z-score | 224 | −0.0031 | 0.747 | −0.0074 | 0.915 |
| Duration of flow reversal during atrial systole, ms | 79 | −0.0011 | 0.087 | −0.0031 | 0.468 |
| Pulmonary vein A-wave duration-for-age z-score | 79 | −0.0123 | 0.473 | −0.0701 | 0.546 |
| R-R interval, ms | 591 | −0.0001 | <.001 | 0.0000 | 0.839 |
| Heart rate, beats/min | 591 | 0.0014 | <.001 | 0.0009 | 0.614 |
EF, ejection fraction; R-R interval, electrocardiogram R-R interval; LV, left ventricular.
Slope for continuous predictors and mean group difference for categorical predictors.
Table 3.
Univariate linear regressions of the S:D ratio raw value and z-score on echocardiographic characteristics at the lateral mitral annulus among normal controls
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 591 | 0.0012 | 0.232 | 0.0456 | <.001 |
| Gender (male vs. female) | 591 | 0.0292 | 0.012 | 0.1784 | 0.034 |
| SBP-for-age z-score | 558 | 0.0214 | <.001 | 0.1276 | 0.003 |
| DBP-for-age z-score | 557 | 0.0121 | 0.035 | 0.0564 | 0.183 |
| EDV-for-BSA z-score | 574 | −0.0185 | 0.002 | −0.0970 | 0.025 |
| ESV-for-BSA z-score | 574 | −0.0314 | <.001 | −0.2409 | <.001 |
| Ventricular mass, g | 575 | 0.0003 | 0.038 | 0.0063 | <.001 |
| Mass-to-volume ratio | 575 | 0.1743 | <.001 | 0.9910 | 0.002 |
| MV-for-age z-score | 575 | 0.0215 | <.001 | 0.1389 | <.001 |
| Sphericity-for-age z-score | 523 | −0.0229 | <.001 | −0.1792 | <.001 |
| VCFc-for-age z-score | 476 | 0.0171 | 0.005 | 0.1240 | 0.005 |
| Peak early velocity (E)-for-age z-score | 459 | −0.0345 | <.001 | −0.2487 | <.001 |
| Peak atrial (A) velocity, m/s | 422 | 0.1371 | 0.008 | 0.5649 | 0.139 |
| Heart rate, beats/min | 591 | 0.0014 | <.001 | 0.0009 | 0.614 |
n Is the total number of observations, not subjects. S:D, systolic and diastolic ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; BSA, body surface area; EDV, end-diastolic volume; ESV, end systolic volume; MV, mass-to-volume ratio; VCFc, velocity of fiber shortening.
Slope for continuous predictors and mean group difference for categorical predictors.
Septal annulus.
Linear associations between the septal S:D coupling ratio and other functional indices with P < 0.1 in normal controls are summarized in Table 4. The results from all tested associations are presented in the appendix (see Table A2). A higher S:D ratio z-score at the septal annulus in healthy children was associated with: older age at echocardiography, male gender, higher systolic blood pressure, larger ventricular dimensions and mass, smaller sphericity index, and smaller peak early diastolic velocity.
Table 4.
Univariate linear regressions of the S:D ratio raw value and z-score at the septal annulus among normal controls
| Outcome = Raw Ratio |
Outcome = Ratio z- Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 590 | −0.0029 | <.001 | 0.0418 | <.001 |
| Gender (male vs. female) | 590 | 0.0249 | 0.010 | 0.1781 | 0.042 |
| SBP-for-age z-score | 557 | 0.0196 | <.001 | 0.1339 | 0.002 |
| DBP-for-age z-score | 556 | 0.0135 | 0.003 | 0.0681 | 0.113 |
| End diastolic volume, ml | 574 | −0.0003 | 0.002 | 0.0046 | <.001 |
| EDV -for- BSA z-score | 573 | −0.0116 | 0.019 | −0.0406 | 0.367 |
| ESV-for-BSA z-score | 573 | −0.0150 | 0.003 | −0.1621 | <.001 |
| Ventricular mass, g | 574 | −0.0003 | 0.014 | 0.0055 | <.001 |
| Mass-to-volume ratio | 574 | 0.1222 | 0.001 | 0.5750 | 0.090 |
| MV-for-age z-score | 574 | 0.0137 | 0.003 | 0.0892 | 0.035 |
| Sphericity-for-age z-score | 522 | −0.0108 | 0.063 | −0.1177 | 0.028 |
| Shortening fraction, % [2D] | 530 | 0.0016 | 0.299 | −0.0236 | 0.093 |
| VCFc-for-age z-score | 476 | 0.0080 | 0.098 | 0.0677 | 0.124 |
| End-systolic meridional fiber stress, g/cm2 | 480 | −0.0009 | 0.014 | 0.0051 | 0.112 |
| End-systolic circumferential fiber stress, g/cm2 | 476 | −0.0004 | 0.016 | 0.0029 | 0.092 |
| Peak early velocity (E) vs. age z-score | 458 | −0.0224 | <.001 | −0.2004 | <.001 |
| Peak atrial (A) velocity, m/s | 422 | 0.1092 | 0.006 | 0.2172 | 0.572 |
| Early deceleration time vs. age z-score | 419 | −0.0064 | 0.215 | −0.0840 | 0.078 |
| Duration of flow reversal during atrial systole, ms | 79 | −0.0007 | 0.070 | 0.0017 | 0.598 |
| Heart rate, beats/min | 590 | 0.0018 | <.001 | −0.0001 | 0.957 |
n Is the total number of observations, not subjects.
Slope for continuous predictors and mean group difference for categorical predictors.
Table A2.
Univariate linear regressions of the S:D ratio raw value and z-score at the septal annulus among normal controls
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 590 | −0.0029 | <.001 | 0.0418 | <.001 |
| Gender (male vs. female) | 590 | 0.0249 | 0.010 | 0.1781 | 0.042 |
| SBP-for-age z-score | 557 | 0.0196 | <.001 | 0.1339 | 0.002 |
| DBP-for-age z-score | 556 | 0.0135 | 0.003 | 0.0681 | 0.113 |
| End diastolic volume, ml | 574 | −0.0003 | 0.002 | 0.0046 | <.001 |
| EDV-for-BSA z-score | 573 | −0.0116 | 0.019 | −0.0406 | 0.367 |
| End systolic volume, ml | 573 | −0.0007 | 0.005 | 0.0113 | <.001 |
| ESV-for-BSA z-score | 573 | −0.0150 | 0.003 | −0.1621 | <.001 |
| Ventricular mass, g | 574 | −0.0003 | 0.014 | 0.0055 | <.001 |
| Mass-for-BSA z-score | 573 | −0.0066 | 0.186 | −0.0396 | 0.379 |
| Mass-to-volume ratio | 574 | 0.1222 | 0.001 | 0.5750 | 0.090 |
| MV-for-age z-score | 574 | 0.0137 | 0.003 | 0.0892 | 0.035 |
| Sphericity index | 522 | −0.1429 | 0.094 | −2.0370 | 0.010 |
| Sphericity-for-age z-score | 522 | −0.0108 | 0.063 | −0.1177 | 0.028 |
| Shortening fraction, % | 530 | 0.0016 | 0.299 | −0.0236 | 0.093 |
| Shortening fraction-for-age z-score | 530 | −0.0083 | 0.119 | −0.0775 | 0.116 |
| Velocity of fiber shortening, circ/s | 476 | 0.2045 | <.001 | 0.6608 | 0.119 |
| VCFc-for-age z-score | 476 | 0.0080 | 0.098 | 0.0677 | 0.124 |
| Ejection fraction, % | 574 | −0.1014 | 0.346 | −0.7649 | 0.433 |
| EF-for-age z-score | 574 | −0.0046 | 0.361 | −0.0307 | 0.497 |
| End-systolic meridional fiber stress, g/cm2 | 480 | −0.0009 | 0.014 | 0.0051 | 0.112 |
| End-systolic circumferential fiber stress, g/cm2 | 476 | −0.0004 | 0.016 | 0.0029 | 0.092 |
| Peak early velocity, m/s | 458 | −0.1802 | <.001 | −0.9902 | <.001 |
| Peak early velocity-for-age z-score | 458 | −0.0224 | <.001 | −0.2004 | <.001 |
| Peak atrial velocity, m/s | 422 | 0.1092 | 0.006 | 0.2172 | 0.572 |
| Peak A-wave velocity-for-age z-score | 420 | 0.0030 | 0.477 | −0.0013 | 0.974 |
| Early deceleration time, ms | 419 | −0.0003 | 0.007 | 0.0010 | 0.424 |
| Deceleration time-for-age z-score | 419 | −0.0064 | 0.215 | −0.0840 | 0.078 |
| Left ventricular flow LV propagation velocity, cm/s | 224 | −0.0001 | 0.682 | 0.0007 | 0.833 |
| LV flow propagation-for-age z-score | 224 | −0.0032 | 0.679 | −0.0041 | 0.954 |
| Duration of flow reversal during atrial systole, ms | 79 | −0.0007 | 0.070 | 0.0017 | 0.598 |
| Pulmonary vein A-wave duration-for-age z-score | 79 | −0.0009 | 0.931 | 0.0221 | 0.801 |
| R-R interval, ms | 590 | −0.0002 | <.001 | 0.0001 | 0.584 |
| Heart rate, beats/min | 590 | 0.0018 | <.001 | −0.0001 | 0.957 |
Slope for continuous predictors and mean group difference for categorical predictors.
Systolic-Diastolic Coupling Ratio in DCM Patients
In comparison to normal controls, at the baseline visit DCM patients had a similar S:D coupling ratio at the lateral mitral annulus (0.58 ± 0.14 vs. 0.54 ± 0.19, P = 0.11) and higher S:D coupling ratio at the septal annulus (0.69 ± 0.13 vs. 0.62 ± 0.12, P = 0.001). At the lateral mitral annulus, the mean S:D coupling ratio z-score of DCM patients (−0.20 ± 1.33) was not different from the normal mean of 0, while at the septal annulus the S:D coupling ratio z-score of DCM was significantly higher than the normal mean (0.87 ± 1.40 vs. 0.00 ± 1.06, P < 0.0001) (Table 2).
Association of the S:D coupling ratio with other functional indexes in DCM.
LATERAL MITRAL ANNULUS.
The results from all tested associations are presented in the appendix (see Table A3). Linear associations between the S:D coupling ratio and other functional indexes in DCM patients at the baseline visit with a P < 0.1 are shown in Table 5. LV EF, age, gender, and blood pressure did not affect the statistical association between S' and E' at the lateral mitral annulus. The S:D coupling ratio at the mitral lateral annulus was positively associated with diastolic blood pressure. LV EDD was positively related to the S:D ratio raw score, while LV mass z-score was positively related to the S:D ratio raw score and z-score. LV flow propagation velocity was negatively associated with S:D ratio raw score and z-score.
Table A3.
Univariate linear regressions of the S:D ratio raw value and z-score at the lateral mitral annulus among DCM patients at baseline
| Outcome = Raw Ratio |
Outcome = Ratio z Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate | P value | Estimate | P value |
| Age at echocardiography, yr | 103 | −0.005 | 0.133 | −0.013 | 0.581 |
| Gender (male vs. female) | 103 | 0.085 | 0.022 | 0.670 | 0.014 |
| SBP-for-age z-score | 102 | 0.0214 | 0.162 | 0.1344 | 0.216 |
| DBP-for-age z-score | 102 | 0.0410 | 0.009 | 0.2704 | 0.016 |
| End diastolic volume, ml | 103 | 0.0000 | 0.948 | 0.0009 | 0.547 |
| EDV-for-BSA z-score | 103 | 0.0151 | 0.010 | 0.1046 | 0.012 |
| End systolic volume, ml | 103 | 0.0001 | 0.659 | 0.0018 | 0.324 |
| ESV-for-BSA z-score | 102 | 0.0220 | 0.004 | 0.1450 | 0.007 |
| Ventricular mass, g | 103 | −0.0001 | 0.716 | 0.0007 | 0.734 |
| Mass-for-BSA z-score | 103 | 0.0331 | <.001 | 0.2188 | <.001 |
| Mass-to-Volume ratio | 103 | 0.0110 | 0.933 | −0.0033 | 0.997 |
| MV-for-age z-score | 103 | 0.0014 | 0.934 | −0.0048 | 0.967 |
| Sphericity index | 103 | 0.1951 | 0.312 | 1.1943 | 0.383 |
| Sphericity-for-age z-score | 103 | 0.0111 | 0.415 | 0.0816 | 0.397 |
| Shortening fraction, % | 103 | −0.0064 | 0.027 | −0.0426 | 0.040 |
| Shortening fraction-for-age z-score | 103 | −0.0248 | 0.009 | −0.1559 | 0.022 |
| Velocity of fiber shortening, circ/s | 102 | −0.1248 | 0.164 | −0.7754 | 0.223 |
| VCFc-for-age z-score | 102 | −0.0162 | 0.056 | −0.0932 | 0.120 |
| Ejection fraction, % | 103 | −0.0038 | 0.023 | −0.0266 | 0.025 |
| EF-for-age z-score | 103 | −0.0136 | 0.071 | −0.1027 | 0.055 |
| End-systolic fiber stress, g/cm2 | 102 | 0.0008 | 0.085 | 0.0064 | 0.051 |
| Peak early velocity, m/s | 82 | −0.0944 | 0.334 | −0.7791 | 0.269 |
| Peak early velocity-for-age z-score | 82 | −0.0133 | 0.449 | −0.1281 | 0.313 |
| Peak atrial velocity, m/s | 82 | 0.2740 | 0.064 | 1.5772 | 0.142 |
| Peak A-wave velocity-for-age z-score | 82 | 0.0249 | 0.104 | 0.1608 | 0.146 |
| Early deceleration time, ms | 82 | 0.0000 | 0.972 | 0.0007 | 0.754 |
| Deceleration time-for-age z-score | 82 | 0.0031 | 0.772 | 0.0249 | 0.746 |
| Left ventricular flow propagation velocity, cm/s | 100 | −0.0018 | 0.073 | −0.0130 | 0.068 |
| LV flow propagation-for-age z-score | 100 | −0.0385 | 0.098 | −0.2897 | 0.078 |
| Duration of flow reversal during atrial systole, ms | 100 | −0.0001 | 0.844 | −0.0004 | 0.846 |
| Pulmonary vein A-wave duration-for-age z-score | 100 | 0.0011 | 0.883 | 0.0022 | 0.967 |
| R-R interval, ms | 103 | 0.0000 | 0.906 | 0.0004 | 0.689 |
| Heart rate, beats/min | 103 | 0.0002 | 0.885 | −0.0043 | 0.654 |
DCM, dilated cardiomyopathy.
Table 5.
Univariate linear regressions of the S:D ratio raw value and z-score at the lateral mitral annulus among dilated cardiomyopathy patients at baseline
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate | P value | Estimate | P value |
| Gender (male vs. female) | 103 | 0.085 | 0.022 | 0.670 | 0.014 |
| DBP-for-age z-score | 102 | 0.0410 | 0.009 | 0.2704 | 0.016 |
| EDV-for-BSA z-score | 103 | 0.0151 | 0.010 | 0.1046 | 0.012 |
| ESV-for-BSA z-score | 102 | 0.0220 | 0.004 | 0.1450 | 0.007 |
| Mass-for-BSA z-score | 103 | 0.0331 | <.001 | 0.2188 | <.001 |
| VCFc-for-age z-score | 102 | −0.0162 | 0.056 | −0.0932 | 0.120 |
| Ejection fraction, % | 103 | −0.0038 | 0.023 | −0.0266 | 0.025 |
| End-systolic fiber stress, g/cm2 | 102 | 0.0008 | 0.085 | 0.0064 | 0.051 |
| Peak atrial velocity, m/s | 82 | 0.2740 | 0.064 | 1.5772 | 0.142 |
| Inflow propagation-for-age z-score | 100 | −0.0385 | 0.098 | −0.2897 | 0.078 |
To further assess these relationships and to incorporate data from serial visits we used a mixed-effects model with piecewise linear terms for the covariate to investigate if the slopes were different before and after the cut-point suggested by the data. Results with P < 0.1 are presented in Table 6. Full results are presented in the appendix. We found that LV mass z-score is significantly associated with S:D ratio z-score when mass z-score is ≥5, but the slope is not different from zero when LV mass z-score is <5. Other variables were not significantly associated with S:D ratio z-score at the mitral lateral annulus.
Table 6.
Mixed model regressions of the S:D ratio z-score at the lateral mitral annulus in dilated cardiomyopathy patients, serial data
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Gender (male vs. female) | 224 | 0.0512 | 0.084 | 0.3794 | 0.069 |
| DBP-for-age z-score | 222 | 0.0228 | 0.031 | 0.1514 | 0.052 |
| Functional status (Ross/NYHA class I, II vs. III, IV) | 224 | 0.0854 | 0.041 | 0.6496 | 0.039 |
| Mass-for-BSA z-score | 223 | 0.0175 | 0.008 | 0.1136 | 0.020 |
| Inflow propagation-for-age z-score | 218 | −0.0254 | 0.048 | −0.2017 | 0.035 |
| Heart rate, beats/min | 224 | −0.0011 | 0.229 | −0.0119 | 0.070 |
n Is the total number of observations, not subjects. NYHA, New York Health Association.
Slope for continuous predictors and mean group difference for categorical predictors.
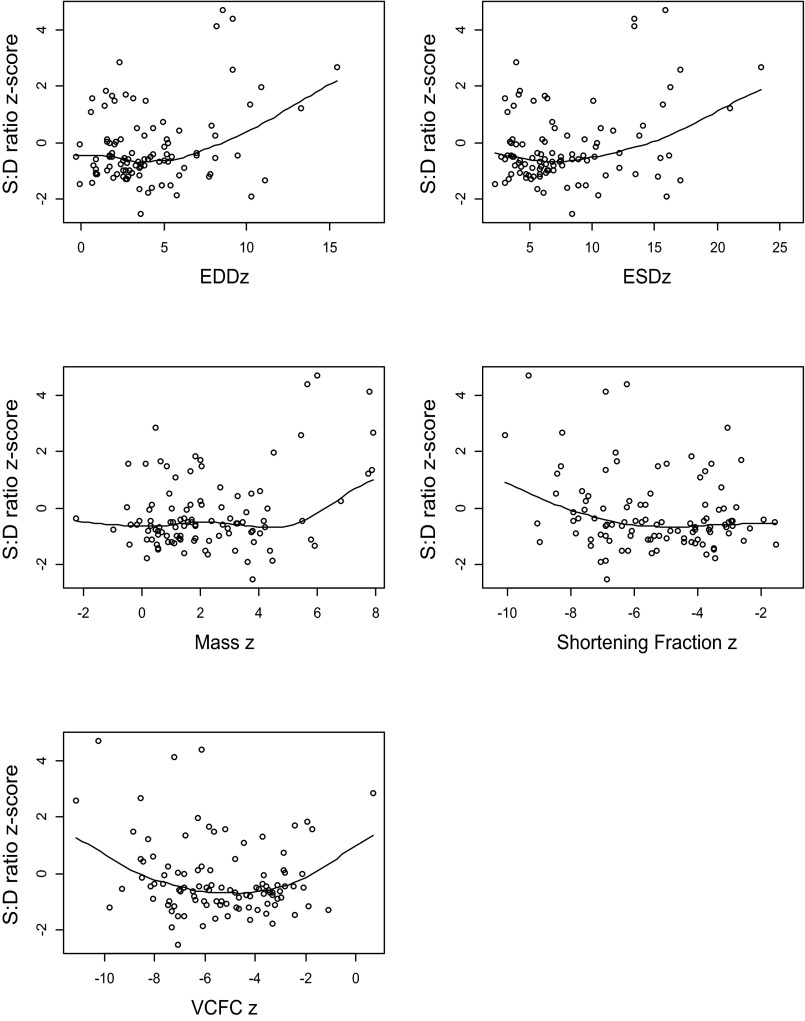
Since scatter plots between the S:D coupling ratio and predictor variables were curvilinear, a quadratic model was also applied to the data. EDD z-score, ESD z- score, ventricular mass z-score, and velocity of fiber shortening z-score showed a significant quadratic relation with the S:D coupling ratio (see Fig. A1). With regard to LV size (EDD z-score and ESD z-score), the model indicated that S:D coupling ratio does not change with LV size until the dimension z-score exceeds ∼5 SD.
Fig. A1.
Systolic:diastolic (S:D) ratio z-score at the lateral mitral annulus vs. parameters of LV size and function. EDDz, end-diastolic dimension z-score; ESDz, end-systolic dimension z-score; VCFCz, velocity of circumferential fiber shortening z-score.
SEPTAL ANNULUS.
Interactions between the S:D coupling ratio and other functional predictors were stronger at the septal than at the lateral mitral annulus. Linear relationships for the baseline visit with P < 0.1 are shown in Table 7 and a mixed-effects model with piecewise linear terms for the covariate incorporating data from all visits are shown in Table 8. The results from all tested associations are presented in the appendix (see Tables A5 and A6). A higher S:D ratio raw score at the septal annulus was associated with larger ventricular dimensions and mass, lower shortening fraction, lower ejection fraction, lower LV flow propagation velocity, shorter duration of flow reversal during atrial systole, and higher heart rate. A piecewise linear model showed that the peak A-wave velocity-for-age z score is significantly associated with septal S:D ratio z-score when peak A-wave velocity z-score is above ≥−0.5 (slope = 0.31, P = 0.018). Likewise, the E/A ratio for age z-score was negatively associated with septal S:D ratio z-score when E/A for age z-score was below 0.5 (slope = −0.55, P = 0.007), but the relationship was not significant when E/A for age z-score is ≥0.5.
Table 7.
Univariate linear regressions of the S:D ratio raw value and z-score at the septal annulus among dilated cardiomyopathy patients at baseline
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 94 | 0.0015 | 0.563 | 0.0631 | 0.016 |
| SBP-for-age z-score | 93 | −0.0200 | 0.078 | −0.2352 | 0.045 |
| End diastolic volume, ml | 94 | 0.0002 | 0.162 | 0.0043 | 0.008 |
| EDV-for-BSA z-score | 94 | 0.0082 | 0.060 | 0.0730 | 0.110 |
| End systolic volume, ml | 94 | 0.0004 | 0.059 | 0.0061 | 0.004 |
| ESV-for-BSA z-score | 93 | 0.0118 | 0.039 | 0.0922 | 0.122 |
| Ventricular mass, g | 94 | 0.0004 | 0.085 | 0.0074 | 0.002 |
| Mass-for-BSA z-score | 94 | 0.0164 | 0.015 | 0.1315 | 0.062 |
| Ejection fraction, % | 94 | −0.0031 | 0.017 | −0.0303 | 0.024 |
Slope for continuous predictors and mean group difference for categorical predictors.
Table 8.
The S:D coupling ratio mixed model regressions of the S:D ratio raw value and z-score at the septal annulus in dilated cardiomyopathy patients
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at baseline echocardiogram, yr | 212 | −0.0002 | 0.920 | 0.0437 | 0.053 |
| DBP-for-age z-score | 210 | −0.0176 | 0.032 | −0.2013 | 0.017 |
| EDV-for-BSA z-score | 212 | 0.0096 | 0.011 | 0.0893 | 0.021 |
| ESV-for-BSA z-score | 209 | 0.0133 | 0.005 | 0.1181 | 0.016 |
| Mass-for-BSA z-score | 212 | 0.0169 | 0.002 | 0.1489 | 0.009 |
| VCFc-for-age z –score | 210 | −0.0092 | 0.044 | −0.0779 | 0.096 |
| Ejection fraction, % | 211 | −0.0030 | 0.002 | −0.0319 | 0.001 |
| Peak atrial (A)-wave velocity-for-age z-score | 166 | 0.0144 | 0.089 | 0.1138 | 0.194 |
| Inflow propagation-for-age z-score | 208 | −0.0336 | <.001 | −0.3538 | <.001 |
| Duration of pulmonary vein A-wave flow reversal-for-age z-score | 208 | −0.0093 | 0.012 | −0.0995 | 0.009 |
| Heart rate, beats/min | 211 | 0.0017 | 0.019 | 0.0092 | 0.204 |
n Is the total number of observations, not subjects.
Slope for continuous predictors and mean group difference for categorical predictors.
Since scatter plots between the S:D coupling ratio and A and E/A ratio were curvilinear, a quadratic model was also applied to the data, with both demonstrating a significant quadratic term.
Comparison of Associations Between S:D Coupling Ratio and Other Parameters of LV Function in DCM vs. Healthy Controls
There were a similar number of associations between the S:D coupling ratio and LV size and function in the DCM patient dataset compared with normal controls for the septal annulus (see Table A7). In both groups, the nonlinear analyses of the component and ratio measures suggest that there is less coupling at the extremes of velocities (lowest values in the DCM patients and highest values in healthy controls).
DISCUSSION
Systolic function contributes to diastolic filling, which in turn influences subsequent systolic contraction through the Frank-Starling effect, even though the coupling between them is not routinely assessed. However, in the presence of myocardial dysfunction, S:D coupling may itself be impaired.
In this exploratory study of a relatively new concept (Fig. 4), we used tissue velocities to investigate the association between systolic and diastolic function in a large group of prospectively collected DCM patients and compared results to a large group of normal controls. The major findings of our study are that:
Fig. 4.
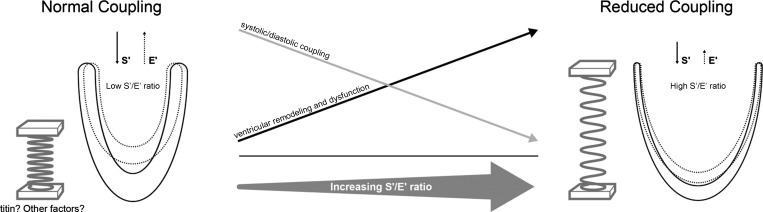
Diagram representing the concepts explored in this study and its main results. On the left a normal LV is depicted with normal systolic and diastolic motion, as assessed by tissue Doppler velocities S' and E', respectively. The solid lines depict the LV in diastole; the dotted lines represent the LV in systole. Based on literature, factors such as the protein titin act as a spring to couple systolic and diastolic function. When the spring's recoil characteristics are intact, depicted as the tightly coiled spring on the left, the ratio between systolic and diastolic velocities (depicted by up and down arrows) is preserved indicating normal systolic-diastolic coupling. In this circumstance, diastolic velocities are relatively close to systolic velocities and consequently the ratio between them (S'/E'; systolic: diastolic coupling index) relatively low. In dilated cardiomyopathy (right), the heart remodels, becomes dysfunctional and with a putative loss of the spring's recoil characteristics (represented by the “stretched” spring on the right). Thus, although S' is lower due to systolic dysfunction, the diastolic coupling is itself impaired resulting in a lower E' for a given S'. This leads to progressive loss of systolic-diastolic coupling and an increasing S'/E' ratio.
1) Systolic and diastolic tissue velocities are positively related.
2) Based on results at the septal annulus, this relationship is impaired in children with DCM compared with that in normal controls.
3) The magnitude of the S:D coupling ratio, a putative index of systolic-diastolic coupling, is related to LV size and global systolic function.
4) Assessment of tissue velocities at the septal annulus appears to be more useful than the lateral annulus when assessing systolic-diastolic coupling.
Relationships Between Systolic and Diastolic Velocities in Normal Children
LV restoring forces, lengthening load, and myocardial relaxation are independent determinants of E' (26). In turn, tension produced in systole, through myocardial contraction and torsion, influences both lengthening load and restoring forces (15).
The relationship between systolic contraction and diastolic filling is through recoil and suction forces (11). The central role of ventricular end-systolic volume in determining coupling is associated with the mechanical action of the heart where release of energy built up in systole creates the early diastolic restoring forces and suction that contribute to rapid early diastolic filling (30). This component of S:D coupling is particularly important in augmenting cardiac output during exercise (11, 24, 31, 32, 36). In disease, the loss of S:D coupling may be particularly detrimental, as the mitral inflow propagation velocity, which reflects in part early diastolic suction, is independently linked to mortality in the same DCM population we studied (23). Likewise, diastole impacts systolic function, primarily through the preload effect of end-diastolic fiber length. Among the factors that determine end-diastolic fiber length, a higher ventricular mass:volume ratio is associated with decreased coupling (higher S:D coupling ratio) (9, 20, 22, 29, 37).
Association Between Systolic and Diastolic Function in Children with DCM
As we previously found in a smaller study (22), systolic and diastolic velocities were related to each other and associated with functional parameters in children with DCM, but coupling was reduced, as reflected by a higher S:D coupling ratio, in DCM patients. As depicted in Fig. 4, lower coupling would lead to a lower E' for a given S' and therefore a higher S:D coupling ratio. The reduction in S:D coupling in DCM patients is likely multifactorial, stemming from both systolic and diastolic dysfunction and from the impaired coupling itself, as suggested by our data. Among the parameters we investigated, LV remodeling is a key candidate to explain decreased coupling, as detailed previously for healthy controls. Our current results indicate that with increasing LV EDD there was a decrease in the strength of S:D coupling. Indeed, studies in patients at the time of cardiopulmonary bypass have shown that improvements in LV function correlate with improvements in LV relaxation and end-systolic volume and are associated with increased early diastolic intraventricular pressure gradients, consistent with improved LV suction and elastic recoil (12). It is also possible that impaired coupling worsens or contributes to LV remodeling and the S:D ratio may in this case be an early marker for LV remodeling in DCM. This requires further investigation. In addition, impaired torsion (6, 27), diastolic dysfunction in various forms (14), and mechanical dyssynchrony (13, 14) may contribute to reduced coupling in DCM patients.
The molecular mediator of S:D coupling is thought to be titin (16). This protein acts as a spring that develops potential energy during systole that is released in early diastole. At the same time, titin protects against excessive sarcomere elongation in late diastole, helping to maintain ventricular dimensions and promote optimal coupling. While our results suggest an important role of LV remodeling in disruption of S:D coupling in DCM patients, the molecular mechanism may be alterations in the titin protein, including shift in isoforms and genetic mutations, among others (2, 18). This requires further study.
Our data suggest that the S:D ratio assessment at the septal annulus may be more useful as an indicator of S:D coupling than the lateral mitral annulus. Likewise, S:D coupling at the septal rather than lateral mitral annulus was more strongly associated with other LV functional parameters. This result was somewhat surprising in that we expected velocities at the lateral mitral annulus to be more strongly associated to LV parameters because the lateral annulus in contrast to the septum is exclusively a LV structure. While the explanation for this result is not obvious, we postulate that septal velocities reflect a combination of right ventricle and LV function and perhaps are more indicative of the sum of RV and LV coupling or perhaps reflect a component of ventricular-ventricular interactions that may be important in DCM (10). Interestingly, lower septal peak systolic TDI velocity z-score and not lateral peak systolic TDI velocity z-score was recently found to be an independent predictor of disease progression in this population (23). Varying activation such as in bundle branch block and other regional wall motion abnormalities may also affect septal vs. lateral velocities.
Clinical Implications
Various echocardiographic parameters have been proposed as associated with outcomes in pediatric DCM. Among these, LV size is important and our results suggest that this may in part be due to loss of S:D coupling (1, 23). Therefore, the S:D coupling ratio warrants further study in relation to clinical outcomes such as brain natriuretic peptide levels and exercise capacity. As diastolic dysfunction is challenging to assess in pediatric DCM (8), this ratio may potentially differentiate between “intrinsic” myocardial diastolic abnormalities and those “secondary” to systolic dysfunction. Treatment of systolic dysfunction would presumably help improve the latter. As this exploratory study did not investigate these concepts, these remain purely conjectures worthy of further study. In this context, it would be interesting to study the relationship in adults with diastolic dysfunction with preserved ejection fraction, an uncommon entity in children (31). Perhaps most importantly, the prognostic value of S:D coupling in clinical practice in the pediatric DCM population requires further study.
Limitations
We did not study the clinical implications of S:D coupling and how its assessment may contribute to functional assessment in general or to prognostication, independent of systolic or diastolic functional assessment alone. Myocardial tissue velocities are segmental in nature and thus velocities sampled at any given point may not reflect the global association between systolic and diastolic function. Radial and rotational motions may also affect systolic-diastolic coupling but were not studied and the S':E' ratio accounts only for longitudinal motion. Likewise, this ratio only assesses early diastole and late diastolic function is not assessed. Tissue velocities are influenced by translational motion that does not reflect S:D coupling. As disease severity increases, tissue Doppler velocities may be harder to measure, with more variability in measurements, which may affect assessment of coupling. Nonetheless, in the VVV study tissue Doppler had acceptable reproducibility in the very same population (5). We did not account for the effect of medications on S:D coupling. In this regard, β-blockers may affect heart rate and thus S:D coupling. Rotational mechanics were not available in the database, and twist-untwist and particularly the rate of twist-untwist may provide a more physiological and/or comprehensive reflection of S:D coupling. Nonetheless, tissue velocities are simpler to obtain and potentially more applicable in routine practice.
Conclusion
In conclusion, systolic and diastolic tissue velocities are positively correlated, reflecting S:D functional coupling. S:D coupling is related to multiple indices of LV size and function but is weaker in DCM as reflected by higher S:D ratios vs. controls, fewer associations between the S:D ratio and parameters of ventricular function and weaker associations between S' and E' with LV remodeling. The S:D coupling ratio, especially when measured at the septal annulus, may be a useful index of coupling performance and warrants further study in relation to outcomes and patient management in children with DCM.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute U01 Grants HL-068269, HL-068270, HL-068279, HL-068281, HL-068285, HL-068292, HL-068290, and HL-068288.
DISCLAIMERS
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.K.F., R.M., L.M.-R., H.T.H., A.N., K.F., K.M.M., K.A., C.C., L.A.S., and S.D.C. conception and design of research; M.K.F., R.M., M.L., L.M.-R., H.T.H., A.N., K.F., K.M.M., K.A., C.C., L.A.S., and S.D.C. interpreted results of experiments; M.K.F., M.L., L.A.S., and S.D.C. prepared figures; M.K.F. drafted manuscript; M.K.F., R.M., M.L., L.M.-R., H.T.H., A.N., K.F., K.M.M., K.A., C.C., L.A.S., and S.D.C. edited and revised manuscript; M.K.F., R.M., M.L., L.M.-R., H.T.H., A.N., K.F., K.M.M., K.A., C.C., L.A.S., and S.D.C. approved final version of manuscript; R.M., M.L., L.A.S., and S.D.C. analyzed data.
ACKNOWLEDGMENTS
The Clinical Trial Registration URL is http://www.clinicaltrials.gov (unique identifier: NCT00123071).
APPENDIX
Figure A1 depicts the systolic:diastolic (S:D) ratio z-score at the lateral mitral annulus vs. parameters of LV size and function. Tables A1, A2, A3, A4, A5, and A6 provide univariate regressions between the S:D ratio z-score and parameters of ventricular function. Table A7 provides the model significance for the S:D ratio at the lateral mitral and septal annulus.
Table A4.
Univariate mixed model regressions of the S:D ratio z-score at the lateral mitral annulus in dilated cardiomyopathy patients
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at baseline echocardiogram, yr | 224 | −0.0004 | 0.877 | 0.0196 | 0.289 |
| Gender (male-for-female) | 224 | 0.0512 | 0.084 | 0.3794 | 0.069 |
| SBP-for-age z-score | 223 | 0.0031 | 0.758 | 0.0162 | 0.828 |
| DBP-for-age z-score | 222 | 0.0228 | 0.031 | 0.1514 | 0.052 |
| Functional status (Ross/NYHA class I, II-for-III, IV) | 224 | 0.0854 | 0.041 | 0.6496 | 0.039 |
| End diastolic volume, ml | 223 | 0.0000 | 0.858 | 0.0012 | 0.315 |
| EDV-for-BSA z-score | 223 | 0.0068 | 0.153 | 0.0437 | 0.205 |
| End systolic volume, ml | 222 | 0.0001 | 0.595 | 0.0018 | 0.234 |
| ESV-for-BSA z-score | 220 | 0.0095 | 0.104 | 0.0579 | 0.173 |
| Ventricular mass, g | 223 | 0.0001 | 0.813 | 0.0019 | 0.263 |
| Mass-for-BSA z-score | 223 | 0.0175 | 0.008 | 0.1136 | 0.020 |
| Mass-to-volume ratio | 223 | 0.1004 | 0.248 | 0.7641 | 0.235 |
| MV-for-age z-score | 223 | 0.0120 | 0.271 | 0.0888 | 0.272 |
| Cardiac index, l/min/m2 [5/6*area*length] | 221 | −0.0043 | 0.707 | −0.0690 | 0.422 |
| Sphericity index | 223 | −0.0403 | 0.768 | −0.5136 | 0.606 |
| Sphericity-for-age z-score | 223 | −0.0029 | 0.762 | −0.0228 | 0.742 |
| Eccentricity index | 223 | 0.2800 | 0.666 | 2.9317 | 0.535 |
| Shortening fraction, % [2D] | 223 | −0.0023 | 0.235 | −0.0162 | 0.250 |
| Shortening fraction-for-age z-score | 223 | −0.0092 | 0.152 | −0.0584 | 0.214 |
| Velocity of fiber shortening, circ/s | 221 | −0.0246 | 0.677 | −0.1434 | 0.740 |
| VCFc-for-age z-score | 221 | −0.0042 | 0.463 | −0.0193 | 0.643 |
| Ejection fraction, % | 222 | −0.0017 | 0.147 | −0.0124 | 0.140 |
| EF-for-age z-score | 222 | −0.0065 | 0.212 | −0.0543 | 0.153 |
| End-systolic fiber stress, g/cm2 | 220 | 0.0002 | 0.600 | 0.0017 | 0.434 |
| Peak early velocity, m/s | 173 | −0.0696 | 0.248 | −0.6087 | 0.172 |
| Peak early velocity-for-age z-score | 173 | −0.0112 | 0.299 | −0.1095 | 0.171 |
| Peak atrial velocity, m/s | 173 | 0.0629 | 0.468 | 0.1257 | 0.844 |
| Peak A-wave velocity-for-age z-score | 173 | 0.0053 | 0.555 | 0.0174 | 0.791 |
| Early deceleration time, ms | 173 | 0.0000 | 0.892 | 0.0001 | 0.924 |
| Deceleration time-for-age z-score | 173 | −0.0007 | 0.911 | −0.0064 | 0.893 |
| Early velocity/atrial velocity (E/A) | 173 | −0.0195 | 0.139 | −0.1313 | 0.179 |
| E/A-for-age z-score | 173 | −0.0115 | 0.168 | −0.0887 | 0.153 |
| Inflow propagation velocity, cm/s | 218 | −0.0011 | 0.045 | −0.0087 | 0.039 |
| Inflow propagation-for-age z-score | 218 | −0.0254 | 0.048 | −0.2017 | 0.035 |
| Myocardial performance index | 212 | 0.0667 | 0.289 | 0.5080 | 0.277 |
| Duration of flow reversal during atrial systole, ms | 218 | 0.0000 | 0.959 | −0.0001 | 0.924 |
| Pulmonary vein A-wave duration-for-age z-score | 218 | 0.0007 | 0.876 | 0.0003 | 0.992 |
| R-R interval, ms | 224 | 0.0001 | 0.336 | 0.0010 | 0.132 |
| Heart rate, beats/min | 224 | −0.0011 | 0.229 | −0.0119 | 0.070 |
Slope for continuous predictors and mean group difference for categorical predictors.
Table A5.
Univariate linear regressions of the S:D ratio raw value and z-score at the septal annulus among DCM patients at baseline
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at echocardiography, yr | 94 | 0.0015 | 0.563 | 0.0631 | 0.016 |
| Gender (male vs. female) | 0.0186 | 0.504 | 0.1684 | 0.562 | |
| SBP-for-age z-score | 93 | −0.0200 | 0.078 | −0.2352 | 0.045 |
| DBP-for-age z-score | 93 | −0.0062 | 0.604 | −0.1044 | 0.399 |
| End diastolic volume, ml | 94 | 0.0002 | 0.162 | 0.0043 | 0.008 |
| EDV-for-BSA z-score | 94 | 0.0082 | 0.060 | 0.0730 | 0.110 |
| End systolic volume, ml | 94 | 0.0004 | 0.059 | 0.0061 | 0.004 |
| ESV-for-BSA z-score | 93 | 0.0118 | 0.039 | 0.0922 | 0.122 |
| Ventricular mass, g | 94 | 0.0004 | 0.085 | 0.0074 | 0.002 |
| Mass-for-BSA z-score | 94 | 0.0164 | 0.015 | 0.1315 | 0.062 |
| Mass-to-Volume ratio | 94 | −0.0030 | 0.976 | 0.0168 | 0.987 |
| MV-for-age z-score | 94 | 0.0002 | 0.991 | −0.0013 | 0.992 |
| Sphericity index | 94 | 0.1501 | 0.288 | 1.0254 | 0.487 |
| Sphericity-for-age z-score | 94 | 0.0135 | 0.176 | 0.1346 | 0.196 |
| Shortening fraction, % | 94 | −0.0030 | 0.192 | −0.0238 | 0.315 |
| Shortening fraction-for-age z-score | 94 | −0.0096 | 0.196 | −0.0568 | 0.465 |
| Velocity of fiber shortening, circ/s | 93 | −0.1001 | 0.145 | −0.8537 | 0.234 |
| VCFc-for-age z-score | 93 | −0.0092 | 0.159 | −0.0546 | 0.421 |
| Ejection fraction, % | 94 | −0.0031 | 0.017 | −0.0303 | 0.024 |
| EF-for-age z-score | 94 | −0.0146 | 0.012 | −0.1613 | 0.008 |
| End-systolic fiber stress, g/cm2 | 93 | 0.0001 | 0.773 | 0.0020 | 0.577 |
| Peak early velocity, m/s | 78 | 0.0823 | 0.281 | 0.7803 | 0.326 |
| Peak early velocity-for-age z-score | 78 | 0.0150 | 0.278 | 0.1158 | 0.421 |
| Peak atrial velocity, m/s | 78 | 0.1894 | 0.116 | 1.1247 | 0.372 |
| Peak A-wave velocity-for-age z-score | 78 | 0.0198 | 0.106 | 0.1572 | 0.218 |
| Early deceleration time, ms | 78 | −0.0001 | 0.588 | −0.0004 | 0.880 |
| Deceleration time-for-age z-score | 78 | −0.0054 | 0.523 | −0.0576 | 0.509 |
| Inflow propagation velocity, cm/s | 92 | −0.0010 | 0.169 | −0.0105 | 0.171 |
| Inflow propagation-for-age z-score | 92 | −0.0243 | 0.156 | −0.2718 | 0.124 |
| Duration of flow reversal during atrial systole, ms | 92 | −0.0001 | 0.674 | −0.0009 | 0.684 |
| Pulmonary vein A-wave duration-for-age z-score | 92 | −0.0020 | 0.716 | −0.0318 | 0.574 |
| R-R interval, ms | 94 | 0.0000 | 0.822 | 0.0008 | 0.483 |
| Heart rate, beats/min | 94 | 0.0002 | 0.871 | −0.0094 | 0.374 |
Slope for continuous predictors and mean group difference for categorical predictors.
Table A6.
Univariate mixed model regressions of the S:D ratio raw value and z-score at the septal annulus in dilated cardiomyopathy patients
| Outcome = Raw Ratio |
Outcome = Ratio z-Score |
||||
|---|---|---|---|---|---|
| Covariate | n | Estimate* | P value | Estimate* | P value |
| Age at baseline echocardiogram, yr | 212 | −0.0002 | 0.920 | 0.0437 | 0.053 |
| Gender (male-for-female) | 212 | 0.0269 | 0.285 | 0.2679 | 0.296 |
| SBP-for-age z-score | 211 | −0.0060 | 0.461 | −0.0891 | 0.289 |
| DBP-for-age z-score | 210 | −0.0176 | 0.032 | −0.2013 | 0.017 |
| Functional status (Ross/NYHA class I, II-for-III, IV) | 212 | −0.0503 | 0.128 | −0.5033 | 0.139 |
| End diastolic volume, ml | 212 | 0.0002 | 0.111 | 0.0042 | 0.003 |
| EDV-for-BSA z-score | 212 | 0.0096 | 0.011 | 0.0893 | 0.021 |
| End systolic volume, ml | 211 | 0.0004 | 0.022 | 0.0061 | <.001 |
| ESV-for-BSA z-score | 209 | 0.0133 | 0.005 | 0.1181 | 0.016 |
| Ventricular mass, g | 212 | 0.0003 | 0.112 | 0.0065 | 0.002 |
| Mass-for-BSA z-score | 212 | 0.0169 | 0.002 | 0.1489 | 0.009 |
| Mass-to-volume ratio | 212 | 0.0300 | 0.684 | 0.3466 | 0.647 |
| MV-for-age z-score | 212 | 0.0031 | 0.737 | 0.0315 | 0.743 |
| Cardiac index, l/min/m2 | 210 | 0.0001 | 0.989 | −0.0663 | 0.480 |
| Sphericity index | 212 | 0.1444 | 0.210 | 1.0753 | 0.362 |
| Sphericity-for-age z-score | 212 | 0.0104 | 0.192 | 0.1059 | 0.195 |
| Eccentricity index | 212 | −0.5826 | 0.284 | −4.1792 | 0.453 |
| Shortening fraction, % | 212 | −0.0031 | 0.047 | −0.0320 | 0.048 |
| Shortening fraction-for-age z-score | 212 | −0.0109 | 0.040 | −0.0982 | 0.071 |
| Velocity of fiber shortening, circ/s | 210 | −0.0918 | 0.050 | −0.9139 | 0.058 |
| VCFc-for-age z-score | 210 | −0.0092 | 0.044 | −0.0779 | 0.096 |
| Ejection fraction, % | 211 | −0.0030 | 0.002 | −0.0319 | 0.001 |
| EF-for-age z-score | 211 | −0.0131 | 0.003 | −0.1513 | <.001 |
| End-systolic fiber stress, g/cm2 | 209 | 0.0001 | 0.720 | 0.0016 | 0.504 |
| Peak early velocity, m/s | 166 | −0.0040 | 0.944 | −0.1806 | 0.762 |
| Peak early velocity-for-age z-score | 166 | −0.0003 | 0.975 | −0.0472 | 0.660 |
| Peak atrial velocity, m/s | 166 | 0.1505 | 0.080 | 0.9013 | 0.309 |
| Peak A-wave velocity-for-age z-score | 166 | 0.0144 | 0.089 | 0.1138 | 0.194 |
| Early deceleration time, ms | 166 | −0.0003 | 0.142 | −0.0021 | 0.253 |
| Deceleration time-for-age z-score | 166 | −0.0093 | 0.126 | −0.1011 | 0.107 |
| Early velocity/atrial velocity | 166 | −0.0158 | 0.329 | −0.0978 | 0.558 |
| E/A-for-age z-score | 166 | −0.0093 | 0.364 | −0.0918 | 0.386 |
| Left ventricular flow propagation velocity, cm/s | 208 | −0.0015 | <.001 | −0.0154 | <.001 |
| LV flow propagation-for-age z-score | 208 | −0.0336 | <.001 | −0.3538 | <.001 |
| Myocardial performance index | 202 | −0.0475 | 0.311 | −0.5026 | 0.303 |
| Duration of flow reversal during atrial systole, ms | 208 | −0.0004 | 0.010 | −0.0038 | 0.012 |
| Pulmonary vein A-wave duration-for-age z-score | 208 | −0.0093 | 0.012 | −0.0995 | 0.009 |
| R-R interval, ms | 211 | −0.0001 | 0.043 | −0.0008 | 0.262 |
| Heart rate, beats/min | 211 | 0.0017 | 0.019 | 0.0092 | 0.204 |
Slope for continuous predictors and mean group difference for categorical predictors.
Table A7.
Model significance for the S:D ratio z-score in dilated cardiomyopathy patients and normal controls at the lateral mitral and septal annulus
| Lateral Mitral Annulus |
Septal Annulus |
|||
|---|---|---|---|---|
| Covariate | DCM | Normal | DCM | Normal |
| Age at baseline echocardiogram, yr | + | + | ||
| Gender (male vs. female) | + | + | ||
| SBP-for-age z-score | + | + | ||
| DBP-for-age z-score | + | – | ||
| End-diastolic short axis dimension, cm | + | + | + | |
| EDD-for-for-BSA z-score | – | |||
| End-systolic short axis dimension, cm | + | + | + | |
| ESD-for–for-BSA z-score | – | + | ||
| End diastolic volume, ml | + | + | + | |
| EDV for BSA\-z-score | – | + | ||
| End systolic volume, ml | + | + | + | |
| ESV for BSA z-score | – | + | – | |
| Ventricular mass, g | + | + | + | |
| Mass-for-BSA z-score | + | + | ||
| Mass-to-volume ratio | + | |||
| MV-for-age z-score | + | + | ||
| Sphericity index | – | – | ||
| Sphericity-age-z | – | – | ||
| Shortening fraction, % | – | |||
| SF-for-age z-score | ||||
| Velocity of fiber shortening, circ/s | + | |||
| VCFc-for-age z-score | + | |||
| Ejection fraction, % | – | |||
| EF-for-age z-score | – | |||
| Peak mitral early velocity (E), m/s | – | – | ||
| Peak mitral E velocity z-score | – | – | ||
| Peak atrial velocity | ||||
| Peak A velocity z-score | ||||
| E-wave deceleration time, ms | ||||
| E-wave deceleration time z-score | ||||
| Inflow propagation velocity, cm/s | – | |||
| Inflow propagation z-score | – | |||
| Duration of flow reversal during atrial systole, ms | – | |||
| Pulmonary vein A-wave duration z-score | – | |||
| R-R interval, ms | – | |||
| Heart rate, beats/min | + | |||
+ or–Indicates P < 0.05.
REFERENCES
- 1.Alvarez JA, Orav EJ, Wilkinson JD, Fleming LE, Lee DJ, Sleeper LA, Rusconi PG, Colan SD, Hsu DT, Canter CE, Webber SA, Cox GF, Jefferies JL, Towbin JA, Lipshultz SE. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. Circulation 124: 814–823, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res 87: 235–240, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Colan S. Normal echocardiographic values for cardiovascular structures. In: Echocardiography in Pediatric and Congenital Heart Disease, edited by Lai WW, Geva T, Mertens L. West Sussex, UK: Wiley-Blackwell, 2009, Appendix 1, p. 765–785. [Google Scholar]
- 4.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol 19: 619–629, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, Chen S, Golding F, Radojewski E, Camitta M, Carboni M, Rychik J, Stylianou M, Tani LY, Selamet Tierney ES, Wang Y, Sleeper LA. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr 25: 842–854 e846, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtois M, Kovacs SJ, Ludbrook PA. Physiological early diastolic intraventricular pressure gradient is lost during acute myocardial ischemia. Circulation 81: 1688–1696, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Doucende G, Schuster I, Rupp T, Startun A, Dauzat M, Obert P, Nottin S. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging 3: 586–594, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Dragulescu A, Mertens L, Friedberg MK. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: problems and limitations. Circ Cardiovasc Imaging 6: 254–261, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, Ayres NA, Bezold LI, O'Brian Smith E, Pignatelli RH. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr 17: 212–221, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Farrar DJ, Chow E, Brown CD. Isolated systolic and diastolic ventricular interactions in pacing-induced dilated cardiomyopathy and effects of volume loading and pericardium. Circulation 92: 1284–1290, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Firstenberg MS, Greenberg NL, Garcia MJ, Thomas JD. Relationship between ventricular contractility and early diastolic intraventricular pressure gradients: a diastolic link to systolic function. J Am Soc Echocardiogr 21: 501–506, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Firstenberg MS, Smedira NG, Greenberg NL, Prior DL, McCarthy PM, Garcia MJ, Thomas JD. Relationship between early diastolic intraventricular pressure gradients, an index of elastic recoil, and improvements in systolic and diastolic function. Circulation 104: I330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg MK, Roche SL, Balasingam M, Stephenson E, Slorach C, Fackoury C, Kantor PF. Evaluation of mechanical dyssynchrony in children with idiopathic dilated cardiomyopathy and associated clinical outcomes. Am J Cardiol 101: 1191–1195, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg MK, Roche SL, Mohammed AF, Balasingam M, Atenafu EG, Kantor PF. Left ventricular diastolic mechanical dyssynchrony and associated clinical outcomes in children with dilated cardiomyopathy. Circ Cardiovasc Imaging 1: 50–57, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Fukuta H, Little WC. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin 4: 1–11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmes M, Trombitas K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res 79: 619–626, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Holubarsch C, Ruf T, Goldstein DJ, Ashton RC, Nickl W, Pieske B, Pioch K, Ludemann J, Wiesner S, Hasenfuss G, Posival H, Just H, Burkhoff D. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation 94: 683–689, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res 114: 1052–1068, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23: 465–495, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Louch WE, Stokke MK, Sjaastad I, Christensen G, Sejersted OM. No rest for the weary: diastolic calcium homeostasis in the normal and failing myocardium. Physiology (Bethesda) 27: 308–323, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Margossian R, Chen S, Sleeper LA, Tani LY, Shirali G, Golding F, Selamet Tierney ES, Altmann K, Campbell MJ, Szwast A, Sharkey A, Radojewski E, Colan SD; Pediatric Heart Network Investigators. The reproducibility and absolute values of echocardiographic measurements of left ventricular size and function in children are algorithm dependent. J Am Soc Echocardiogr 28: 549–558 e541, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed A, Mertens L, Friedberg MK. Relations between systolic and diastolic function in children with dilated and hypertrophic cardiomyopathy as assessed by tissue Doppler imaging. J Am Soc Echocardiogr 22: 145–151, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Molina KM, Shrader P, Colan SD, Mital S, Margossian R, Sleeper LA, Shirali G, Barker P, Canter CE, Altmann K, Radojewski E, Tierney ES, Rychik J, Tani LY. Predictors of disease progression in pediatric dilated cardiomyopathy. Circ Heart Fail 6: 1214–1222, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, Garcia MJ, Greenberg NL, Thomas JD. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation 113: 2524–2533, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 294: H505–H513, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, Edvardsen T, Smiseth OA. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation 119: 2578–2586, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Pacileo G, Baldini L, Limongelli G, Di Salvo G, Iacomino M, Capogrosso C, Rea A, D'Andrea A, Russo MG, Calabro R. Prolonged left ventricular twist in cardiomyopathies: a potential link between systolic and diastolic dysfunction. Eur J Echocardiogr 12: 841–849, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation 90: 2772–2779, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Roberson DA, Cui W, Chen Z, Madronero LF, Cuneo BF. Annular and septal Doppler tissue imaging in children: normal z-score tables and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr 20: 1276–1284, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Robinson TF, Factor SM, Sonnenblick EH. The heart as a suction pump. Sci Am 254: 84–91, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Rovner A, Greenberg NL, Thomas JD, Garcia MJ. Relationship of diastolic intraventricular pressure gradients and aerobic capacity in patients with diastolic heart failure. Am J Physiol Heart Circ Physiol 289: H2081–H2088, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Rovner A, Odabashian J, Vendetti A. Diastolic suction is a major determinant of aerobic exercise capacity in heart failure patients. J Am Soc Echocardiogr 15: 479, 2002. [Google Scholar]
- 33.Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J 48: 286–301, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Schwinger RH, Bohm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res 74: 959–969, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Vahl CF, Timek T, Bonz A, Kochsiek N, Fuchs H, Schaffer L, Rosenberg M, Dillmann R, Hagl S. Myocardial length-force relationship in end stage dilated cardiomyopathy and normal human myocardium: analysis of intact and skinned left ventricular trabeculae obtained during 11 heart transplantations. Basic Res Cardiol 92: 261–270, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Vlassak I, King LM Greenberg NL, Firstenberg MS, Thomas JD, Garcia MJ. Early apical relaxation increases ventricular suction during exercise in normal volunteers. J Am Coll Cardiol 37: 442, 2001. [Google Scholar]
- 37.Yip GW, Zhang Y, Tan PY, Wang M, Ho PY, Brodin LA, Sanderson JE. Left ventricular long-axis changes in early diastole and systole: impact of systolic function on diastole. Clin Sci (Lond) 102: 515–522, 2002. [PubMed] [Google Scholar]