Abstract
Summary
Objective Growth hormone (GH) replacement may increase bone mineral density (BMD) in GH-deficient (GHD) adults. The goal of this study was to identify predictors of BMD response to GH replacement in GH na ve adults.
Design and measurements
This was a retrospective analysis of data extracted from KIMS (Pfizer International Metabolic Database), an international pharmacoepidemiological survey of adult GHD patients from 31 countries.
Patients
A total of 231 GH naive adults were identified (115 women and 116 men) who had BMD measured on the same densitometer in the lumbar spine (LS) and/or femoral neck (FN) both at baseline and after 4 years of GH replacement.
Results
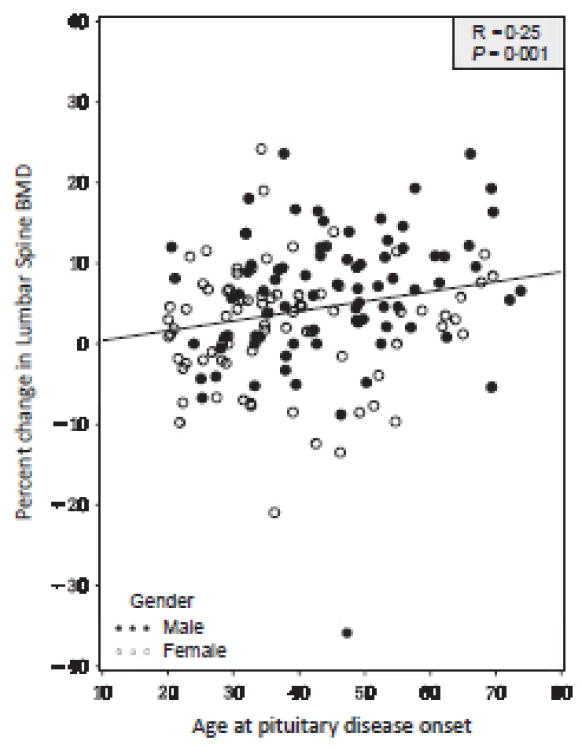
After 4 years, there was a median (10th, 90th percentile) 4.6% (−5.2%, 12.2%) increase in LS BMD over baseline (P = 00001). There was a positive correlation between per cent change in LS BMD and age at the onset of pituitary disease (r = 025, P = 0001). There was no change in FN BMD over baseline [0.0% (−7.3%, 8.5%)]. On multivariate analysis, older age at the onset of pituitary disease predicted a greater increase in LS BMD on GH replacement (r = 0.55, P < 0.0001).
Conclusions
In a population of GH na ve adults, GH replacement led to a significant increase in LS BMD over baseline, but no change in FN BMD. The potential for greater BMD improvement on GH replacement therapy in adults with disease of later onset should be considered when making treatment decisions in this patient population.
Introduction
Growth hormone (GH) replacement may increase bone mineral density (BMD) in adults with GH deficiency (GHD). (1–6) However, it remains unclear if specific demographic, clinical or endocrine factors predict BMD response to GH replacement.
Some studies have suggested that GHD men receiving GH replacement may show a greater increase in BMD than women.(1,6) However, this is not a universal finding.(5) Adult GHD patients frequently have additional pituitary hormone deficiencies, leading to target gland hormone deficiencies that may affect BMD either directly or indirectly (through the effects of respective replacement therapies).(7–10)
The hypothesis of this study was that demographic, clinical and endocrine factors may influence BMD response to GH replacement in adult GHD patients. To test this hypothesis, KIMS (Pfizer
International Metabolic Database) was queried to identify GH na ve adults with GHD and characterize factors associated with BMD response to GH replacement for 4 years.
Subjects and methods
Subjects
The KIMS database11 (including over 16 000 patients) was searched to identify adult-onset GHD patients who were over 20 years old at diagnosis of pituitary disease, were GH na ve at study entry and had BMD measured in the posterior–anterior lumbar spine (LS) and/or femoral neck (FN) on the same densitometer by dual energy X-ray absorptiometry (DXA), both at baseline and after 4 years of GH replacement (on therapy for ≥90% of this period). The diagnosis of GHD was based on stringent criteria as described previously. (12–14) Patients taking medications that could affect BMD, including bisphosphonates (N = 13) and antiepileptic agents (N = 11), were identified. After excluding these patients, the search included 231 adult GHD subjects in two overlapping subgroups, including 157 patients with available data on LS BMD and 187 patients with available data on FN BMD.
Informed consent was obtained at each participating centre at the time of entry into the study. The principles of the Declaration of Helsinki were adhered to during the study. (15)
Methods
Data extracted from the database included subjects’ age (at the onset of pituitary disease, diagnosis of GHD and study entry), gender, body mass index (BMI), cause of hypopituitarism, history of pituitary surgery or radiation therapy, peak GH response on stimulation testing, insulin-like growth factor 1 (IGF-1) standard deviation scores (SDS), presence of additional pituitary hormone deficiencies, sex steroid replacement status, GH replacement dose, hydrocortisone equivalent replacement dose (calculated as previously described),(16) use of medications that may affect BMD (including bisphosphonates and antiepileptic agents), baseline BMD Z scores, baseline and 4-year BMD values. Absolute BMD values were used to calculate per cent BMD change over baseline. All data had been submitted for entry in the database by individual centres where patients received care. Serum IGF-1 levels were measured centrally and used to calculate IGF-1 SDS.
Serum IGF-1 levels were measured at Kabi Pharmacia (Stockholm, Sweden) between 1994 and October 1997. Thereafter, serum IGF-1 levels were assayed at Sahlgrenska University Hospital (Gothenburg, Sweden). Between 1994 and November 2002, a radioimmunoassay (RIA) was used to measure serum IGF-1 levels after acid-ethanol precipitation to remove IGF binding proteins, as previously described (Nichols Institute, San Juan Capistrano, CA, USA).(17) Subsequently, the Nichols Advantage system (chemiluminescence immunoassay) was used until September 2006. Thereafter, Immulite 2500 has been used and is the currently employed method (Diagnostic Products Corp. Siemens, Deerfield, IL, USA). To calculate IGF-1 SDS, the following formulae have been used: SDS = [ln (IGF-1) – (5.95 – 0.0197 x age)] (between 1994 and 1997); SDS = [ln (IGF-1) – (15.92 – 0.0146 x age)/0.272] (between 1997 and 2002); and (from 2002 onwards) based on published data by Brabant et al. (18)
Statistical analyses
The Student’s t-test was used to compare normally distributed continuous data and the Wilcoxon rank sum test was used to compare nonparametric continuous variables. Proportions were compared using the chi-square test. Data on absolute and percent changes in BMD in the LS and FN over 4 years were analyzed using the sign rank test.
Univariate, nonparametric analyses were performed to examine the association between change in BMD over baseline and age or replacement dose (glucocorticoid or levothyroxine), as well as changes in BMD in patient subgroups (stratified by gender, sex steroid replacement status, baseline Z score). In addition, nonparametric multivariate analyses were conducted to examine the association between possible predictors and (per cent or absolute) BMD change over baseline. Nominal variables were assigned numerical codes for the purpose of analysis. Possible predictors examined include age at the onset of pituitary disease, age at GHD diagnosis, age at study entry, estimated duration of GHD, gender, BMI, presence of corticotropin, thyrotropin or gonadotropin deficiency, sex steroid replacement status, presence of diabetes insipidus, peak GH response on stimulation testing, IGF-1 SDS at study entry, baseline BMD at study entry, GH dose and history of radiation therapy.
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, Inc., Cary, NC, USA). Data were expressed as median (10th, 90th percentile) or mean ± standard deviation (SD). P values less than 0.05 were considered statistically significant.
Results
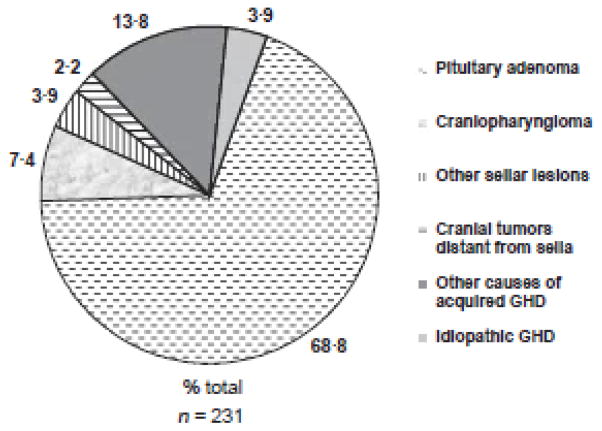
The search identified 231 adult GHD subjects, all GH na ve at the time of KIMS entry. There were 115 women (49.8%) and 116 men (50.2%). Most study subjects were Caucasian and were enrolled in European centres. Additional characteristics of the study population are shown in Table 1. Glucocorticoid replacement dose (expressed as hydrocortisone equivalent) was 21.9 mg ± 7.5 mg daily (data available for 137 patients) and levothyroxine replacement dose was 1.5 ± 1.1 μg/kg daily (data available for 117 patients). Among women on sex steroid replacement, 65.1% were taking oral oestrogen and 12.7% were using transdermal oestrogen. The route of oestrogen replacement was not known in 22.2% of women. Data on the causes of hypopituitarism are shown in Fig. 1. The insulin tolerance test was used as the GH stimulus in 175 subjects (76.1%). Other GH stimulation tests [including glucagon, arginine or growth hormone releasing hormone (GHRH)-arginine] or the presence of multiple pituitary hormone deficiencies with low IGF-1 levels was used to diagnose GHD in the remainder of the study population, based on stringent diagnostic criteria (Table 2). (12–14)
Table 1.
Baseline characteristics of the study population
| Variable | |
|---|---|
| Population size (n) | 231 |
| Age at diagnosis of pituitary disease (years) | 41·3 (25·2, 62·2) |
| Age at diagnosis is of GHH (years) | 46·4 (31·0.64·9) |
| Age at KIMS entry (years) | 48·8 (33·9, 66·1) |
| Gender [women/men (%)] | 115/116 (49·8, 50·2) |
| History of pituitary surgery [n(%)] | 161 (69·7) |
| History of cranial radiation therapy [n [%)] | 64 (27·7) |
| IGF-1 SDS | −1·5 (−4·0, −0·1) |
| Corticotropin deficiency [n (%)] | 162 (70·1) |
| Thyrotropin deficiency [n (%)] | 162 (10·1) |
| Gonadotropin deficiency [n (%)] | 191 (82·7) |
| Sex steroid replacement, status (def/repl/suff) [n(%)] | 39/152/40 (16·9, 65·8, 17·3)† |
| Central diabetes insipidus [n (%)] | 43 (18·6) |
| Number of additional pituitary hormone deficiencies [n(%)] | None 20 (8·7) |
| One 41 (17·8) | |
| Two 29 (12·5) | |
| Three 108 (46·7) | |
| Four 33 (14·3) | |
| Hydrocortisone equivalent dose [mg/day]* | 20·0 (12·0, 30·0) |
| Lumbar spine Z score | −0·5 (−2·0, 1·6) |
| Femoral neck Z score | −0·3 (−1·5, 1·1) |
Fig. 1.
Causes of growth hormone deficiency (GHD) in the study population (per cent total), adapted a previously defined Classification List.34
Table 2.
Diagnostic criteria for growth hormone deficiency
| Test | Diagnostic cutpoint | |
|---|---|---|
| Insulin tolerance test | Peak GH < 3 μg/l | |
| Glucagon stimulation test | Peak GH < 3 μg/l | |
| GHRH + arginine stimulation test | If BMI < 25 kg/m2 | Peak GH < 11· 5 μg/l |
| If BML 25–30 kg/m2 | Peak GH < 8 μg/l | |
| If BML > 30 kg/m2 | Peak GH < 4·2 μg/l | |
| Arginine stimulation test IGF-1 | Peak GH < 0·4 μg/l IGF-1 SDS <–2 and ≥3 additional pituitary hormone deficiencies |
Growth hormone replacement was prescribed according to local clinical practice, and was initiated at a median dose (10th and 90th percentile) of 0.2 mg/day (0.1, 0.3). During the last year included in the study period (year 4), the median (10th and 90th percentile) GH dose had been titrated to 0.4 mg/day (0.2, 0.8) with median (10th and 90th percentile) serum IGF-1 SDS of 0.8 (−1.1, 2.2). After 4 years of GH replacement, there was a median 4.6% (−5.2%, 12.2%) increase in LS BMD over baseline in the study population (N = 157, P = 0.0001). There was a positive correlation between per cent change in LS BMD and age at the onset of pituitary disease (r = 0.25, P = 0.001, Fig. 2). There was no change in FN BMD over baseline [0.0% (−7.3%, 8.5%)] in the study population (N = 187, P = NS). Absolute changes in BMD closely paralleled per cent changes in the study population or subgroups (data not shown).
Fig. 2.
Association between per cent change in lumber spine (LS) bone mineral density (BMD) over baseline and age at the onset of pituitary disease.
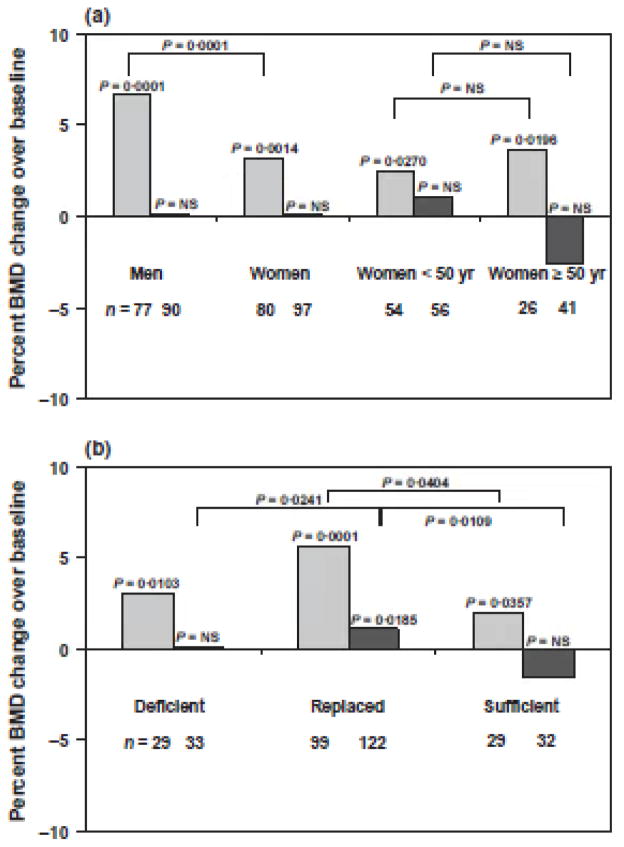
Data on per cent BMD changes over baseline, stratified by gender, are shown in Fig. 3a. In men, there was a median (10th and 90th percentile) 6.7% (−4.1%, 15.2%) increase in LS BMD over baseline after 4 years of GH replacement (P = 0.0001, Fig. 3a). In women, there was a median (10th and 90th percentile) 3.2% (−7.5%, 9.9%) increase in LS BMD over baseline over the same study period (P = 0.0014).
Fig. 3.
The per cent increase in LS BMD over baseline was greater in men than in women (P = 0.0001). Of note, men were older than women at the time of onset of pituitary disease [median (10th and 90th percentile)] [men: 45.7 years (29.4, 64.4), women: 35.8 years (22.8, 58.7), P = 0.0002] as well as at study entry [men: 51.3 years (35.4, 66.9), women: 46.4 years (33.8, 63.3), P = 0.0180]. Despite higher median (10th and 90th percentile) GH doses being prescribed to women than men [women: 0.4 mg/day (0.2, 1.0), men: 0.3 mg/day (0.1, 0.5), P = 0.0001], median (10th and 90th percentile) serum IGF-1 SDS were higher in men than in women [men: 0.9 (−0.6, 2.4), women: 0.5 (−1.4, 1.8), P = 0.0166] during the last year of the study. There was no difference in LS BMD per cent increase over baseline between women <50 years and ≥50 years old at study entry (P = NS) nor between women on oral and transdermal oestrogen replacement (data not shown; P = NS).
There was no change in FN BMD over baseline in study subjects of either gender over the 4-year period (Fig. 3a). In addition, there were no significant per cent changes in FN BMD over baseline in women <50 years or ≥50 years at study entry. The difference in FN BMD per cent change over baseline between women <50 years (who showed a trend towards an increase in FN BMD) and ≥50 years old (who showed a trend towards a decrease in FN BMD) was not statistically significant (P = NS).
Data on per cent BMD changes over baseline, stratified by sex steroid replacement status (deficient: sex steroid deficient; replaced: sex steroid replaced; sufficient: endogenously sex steroid sufficient), are shown in Fig. 3b. There were statistically significant per cent (median, 10th and 90th percentile) increases in LS BMD over baseline in deficient [3.0% (−6.7%, 13.7%), P = 0.0103], replaced [5.6% (−5.2%, 14.6%), P = 0.0001] and sufficient [2.0% (−5.4%, 9.6%), P = 0.0357] subjects. Of note, there was a statistically significant difference in LS BMD per cent increase over baseline between replaced and sufficient groups (P = 0.0404), but not between deficient and replaced or deficient and sufficient groups (P = NS).
There was a median (10th and 90th percentile) 1.2% (−5.6%, 10.5%) increase in FN BMD over baseline in the replaced group (P = 0.0185, Fig. 3b). There were no significant changes in FN BMD over baseline in the deficient and sufficient groups. Of note, there were statistically significant differences in FN BMD per cent change over baseline between deficient and replaced (P = 0.0241) or between replaced and sufficient (P = 0.0109) groups [but not between deficient and sufficient groups (P = NS)].
Univariate correlation analyses were conducted to investigate the possibility of a deleterious effect of either glucocorticoid or thyroid hormone replacement on BMD response to GH replacement. There was no association between per cent change in LS BMD (or per cent change in FN BMD) over baseline and either glucocorticoid or levothyroxine replacement dose (P = NS).
To investigate the influence of baseline Z score on BMD response to GH replacement, data on change in LS BMD over baseline were stratified by baseline Z scores (<−2.0, between −2.0 and −1.0, between −1.0 and 0, >0). There was no statistically significant difference in per cent (or absolute) change in LS BMD over baseline, when stratified by baseline LS Z score (Table 3).
Table 3.
Per cent charge in lumber spine (LS) and femoral neck (FN) bone mineral density (BMD) stratified by baseline Z score (as available)
| Baseline Z sore | Per cent change in LS BMD over baseline [n] | p value (charge in LS BMD over baseline) | Per cent charge in FN BMD over baseline [n] | p value (charge in FN BMD over baseline) |
|---|---|---|---|---|
| < −2·0 | −2·0 (−35·9, 23·6)* [5] | NS | 17·2 [1] | Not applicable |
| Between −2·0 and −1·0 | 6·7 (−2·4, 9·8)* [23] | 0·0002 | 3·5 (−6·6, 13·9)† [25] | 0·052 |
| Between −1·0 and 0 | 6·2 (−4·8, 19·3)* [29] | 0·0001 | 0 (−6·1, 7·9) [53] | NS |
| > 0 | 4·3 (−4·1, 11·9)* [38| | 0·0001 | 0 (−11·2, 6·3) [50] | NS |
Data are shown as median (10th and 90th percentiles).
BMD, bone mineral density; FN, femoral neck; LS, lumber spine; NS, not significant.
Difference between these subgroups was not statistically significant (p = NS).
Difference between this subgroup and all others was statistically significant (p < 0·05).
In the FN, there was a 3.5% (−6.6%, 13.9%) increase in BMD over baseline (P = 0.052) in the subgroup of 25 patients with baseline Z score between −2.0 and −1.0 (Table 3). Expressed as absolute change in FN BMD over baseline, there was a 0.03 g/cm2 (−0.05, 0.11) in the same patient subgroup (P = 0.040).
Stepwise multivariate analyses were conducted to identify independent predictors of per cent change in LS BMD over baseline. Age at the onset of pituitary disease was positively associated with greater increase in LS BMD over baseline (r = 0.55, P < 0.0001). A higher GH dose was also positively associated with LS BMD change, but the statistical significance of this association was borderline (r = 0.21, P = 0.0787). The same predictors were identified in analyses where absolute change in LS BMD over baseline was the dependent variable (data not shown). Of note, neither subject gender nor sex steroid replacement status remained independent predictors of change in LS BMD over baseline on multivariate analysis.
Discussion
In this study, the effects of 4 years of GH replacement on BMD were examined in GH na ve adults using data extracted from KIMS, a large international pharmacoepidemiological survey of GH replaced adults. In this study population, there was a significant increase in BMD over baseline in the LS. This finding is in broad agreement with several other studies.(2,3,6,19–21) However, it may be noted that it is still not universally agreed upon that GH replacement in adult GHD leads to increased BMD.(22) Although this study did not include untreated control subjects, it is unlikely that LS BMD would spontaneously increase to the extent reported herein in an adult population almost entirely past the age of peak bone mass (90% of subjects being −33.9 years old at study entry).(23,24) Moreover, study subjects were not taking bisphosphonates or other medications specifically used to increase BMD (by study design). The magnitude of the increase in LS BMD is comparable to the effect of bisphosphonates, albeit this is only based on historical comparisons. Overall, the present findings affirm a relevant role of GH replacement with regard to BMD in the LS. Although there was no change in FN BMD in the entire study population, there was a positive trend in FN BMD in women <50 years and a negative trend in FN BMD in women 50 years at study entry, the latter possibly reflecting menopause- induced bone loss. In addition, there was an increase in FN BMD in the subgroup of patients with baseline Z score between −2.0 and −1.0, albeit of marginal statistical significance. The absence of an overall change in FN BMD over baseline in women and men in this study remains of unclear significance. One may speculate that a decrease in FN BMD might have occurred without GH replacement; however, this cannot be ascertained in the absence of an untreated control group. The FN contains a higher proportion of compact or cortical bone than the LS and may respond differently to pharmacological interventions, including GH. For example, the LS, rich in trabecular bone, responds to a greater degree than the FN, rich in cortical bone, to the anabolic agent teriparatide.(25) Growth hormone replacement activates bone turnover, including both bone formation and resorption, with the net effect of GH replacement on BMD depending on the balance between these two effects.(26–31) Some studies have shown an increase in FN BMD in response to GH replacement.1,19 However, another study of GH replacement did not show an increase in total hip BMD.6 In a recently published long-term study of GH replacement, there was a transient increase in FN BMD, which returned to baseline by the end of study period, possibly reflecting opposing effects, including a positive effect of GH replacement on BMD and age-related bone loss.92)
In this study, there was a greater improvement in LS BMD in association with older age at the onset of pituitary disease, a finding that remained robust on multivariate analysis. This observation has not been previously reported to the authors’ knowledge. In another study, comparable benefits with regard to gain in LS BMD were reported in older as well as younger GHD adults.93) The present study suggests that patients with pituitary disease of later onset may have substantial improvement in LS BMD on GH replacement, an observation to be considered when making treatment decisions in older adult GHD patients. However, these data cannot be extrapolated to patients >70 years of age, as very few such patients were included in the study.
The influence of gender on BMD response to GH replacement is another area of controversy. In some studies, women appeared to show less or no effect of GH replacement on BMD in comparison with men.91,6,19) In contrast, women appeared to benefit to the same extent as men with regard to BMD response to GH replacement in another publication.(5) In this study, men showed a greater increase in LS BMD over baseline than women, an association that did not persist on multivariate analysis. It is possible that some of the discrepancies between studies may be explained on the basis of resistance to GH replacement in women receiving oral sex steroid replacement.(32,33) In agreement with these considerations, women had lower median IGF-1 SDS than men in the last year of this study, despite receiving higher median GH doses. These observations are clinically relevant and affirm the importance of adequate GH dosing, particularly in women receiving oral sex steroid replacement.
In this study, subjects on sex steroid replacement appeared to show a greater increase in LS BMD over baseline, a finding that did not persist on multivariate analysis. Only sex steroid replaced subjects showed a fairly small per cent increase in FN BMD over baseline. The effect of sex steroid replacement on GH-replaced adults also represents an area of controversy.(5,19) This study’s findings suggest that sex steroid replacement may not be essential to achieve improvements in LS BMD in response to GH replacement. However, sex steroid replacement should still be considered for other benefits and might optimize skeletal outcomes in this population.
Strengths of this study include the relatively large size of the study population and length of observation period, stringent definition of GHD, inclusion of strictly GH na ve subjects and the requirement that both BMD measurements be performed on the same densitometer. The study has limitations inherent to its retrospective design, which necessitates the inclusion of patients with available data in the study population. In addition to the lack of an untreated control group, it may be noted that information on exercise, cigarette smoking, alcohol, calcium and vitamin D intake was not available. As most study subjects were Caucasian, the findings of this study may not fully apply to patients of diverse racial and ethnic backgrounds.
In conclusion, GH replacement may increase LS BMD over baseline in GH na ve adults. Based on this study’s findings, the effect of GH replacement on FN BMD is less clear. Patients who are older at the time of onset of pituitary disease may show a greater improvement in LS BMD in response to GH replacement. These observations may be considered when making treatment decisions in this patient population.
Acknowledgments
The authors would like to express their gratitude to all clinicians who submitted the primary data on their patients to the KIMS database. KIMS is sponsored by Pfizer, Inc. N.A.T., A.H.H., S.L.G., D.M.C. and B.M.K.B. were not compensated for their contributions to this manuscript.
Footnotes
Competing interests
N.A.T. has been a recipient of research funding from Pfizer and Ipsen, and received consulting fees from Pfizer; spouse is an employee of Pfizer. A.H.H. has been a recipient of lecture fees from Novartis, Ipsen and Pfizer, and has served as consultant to Ipsen and Pfizer. S.L.G. has been a recipient of research funding from Eli Lilly, Warner Chilcott, Tarsa, and has consulted and served on an advisory board for Amgen and Merck. D.K., P.J.J. and M.K-H. are full-time employees of Pfizer. B.M.K.B. has been a recipient of research funding from Pfizer, Novo Nordisk and Serono, has served on an advisory board with consulting fees from Pfizer and Novo Nordisk.
References
- 1.Drake WM, Rodriguez-Arnao J, Weaver JU, et al. The influence of gender on the short and long-term effects of growth hormone replacement on bone metabolism and bone mineral density in hypopituitary adults: a 5-year study. Clinical Endocrinology (Oxford) 2001;54:525–532. doi: 10.1046/j.1365-2265.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 2.Elbornsson M, Gotherstrom G, Bosaeus I, et al. Fifteen years of GH replacement increases bone mineral density in hypopituitary patients with adult-onset GH deficiency. European Journal of Endocrinology. 2012;166:787–795. doi: 10.1530/EJE-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbornsson M, Gotherstrom G, Franco C, et al. Effects of 3-year GH replacement therapy on bone mineral density in younger and elderly adults with adult-onset GH deficiency. European Journal of Endocrinology. 2012;166:181–189. doi: 10.1530/EJE-11-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fideleff HL, Boquete HR, Stalldecker G, et al. Comparative results of a 4-year study on cardiovascular parameters, lipid metabolism, body composition and bone mass between untreated and treated adult growth hormone deficient patients. Growth Hormone & IGF Research. 2008;18:318–324. doi: 10.1016/j.ghir.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Gotherstrom G, Bengtsson BA, Bosaeus I, et al. Tenyear GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. European Journal of Endocrinology. 2007;156:55–64. doi: 10.1530/eje.1.02317. [DOI] [PubMed] [Google Scholar]
- 6.Snyder PJ, Biller BM, Zagar A, et al. Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiency. Journal of Bone and Mineral Research. 2007;22:762–770. doi: 10.1359/jbmr.070205. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Annals of Internal Medicine. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Rodriguez E, Stewart PM, Cooper MS. The pituitary-adrenal axis and body composition. Pituitary. 2009;12:105–115. doi: 10.1007/s11102-008-0098-2. [DOI] [PubMed] [Google Scholar]
- 9.Debono M, Ross RJ, Newell-Price J. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. European Journal of Endocrinology. 2009;160:719– 729. doi: 10.1530/EJE-08-0874. [DOI] [PubMed] [Google Scholar]
- 10.Venken K, Callewaert F, Boonen S, et al. Sex hormones, their receptors and bone health. Osteoporosis International. 2008;19:1517–1525. doi: 10.1007/s00198-008-0609-z. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez LP, Koltowska-Haggstrom M, Jonsson PJ, et al. Registries as a tool in evidence-based medicine: example of KIMS (Pfizer International Metabolic Database) Pharmacoepidemiology and Drug Safety. 2008;17:90–102. doi: 10.1002/pds.1510. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. European Journal of Endocrinology. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 13.Tritos NA, Greenspan SL, King D, et al. Unreplaced sex steroid deficiency, corticotropin deficiency, and lower IGF-I are associated with lower bone mineral density in adults with growth hormone deficiency: a KIMS database analysis. Journal of Clinical Endocrinology and Metabolism. 2011;96:1516–1523. doi: 10.1210/jc.2010-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tritos NA, Hamrahian AH, King D, et al. A longer interval without GH replacement and female gender are associated with lower bone mineral density in adults with childhoodonset GH deficiency: a KIMS database analysis. European Journal of Endocrinology. 2012;167:343–351. doi: 10.1530/EJE-12-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riis P. Thirty years of bioethics: the Helsinki Declaration 1964–2003. New Review of Bioethics. 2003;1:15–25. doi: 10.1080/1740028032000131396. [DOI] [PubMed] [Google Scholar]
- 16.Filipsson H, Monson JP, Koltowska-Haggstrom M, et al. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. Journal of Clinical Endocrinology and Metabolism. 2006;91:3954–3961. doi: 10.1210/jc.2006-0524. [DOI] [PubMed] [Google Scholar]
- 17.Underwood LE, Attie KM, Baptista J. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebocontrolled study. Journal of Clinical Endocrinology and Metabolism. 2003;88:5273–5280. doi: 10.1210/jc.2003-030204. [DOI] [PubMed] [Google Scholar]
- 18.Brabant G, von zur Muhlen A, Wuster C, et al. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Hormone Research. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 19.Rota F, Savanelli MC, Tauchmanova L, et al. Bone density and turnover in young adult patients with growth hormone deficiency after 2-year growth hormone replacement according with gender. Journal of Endocrinological Investigation. 2008;31:94–102. doi: 10.1007/BF03345574. [DOI] [PubMed] [Google Scholar]
- 20.Arwert LI, Roos JC, Lips P, et al. Effects of 10 years of growth hormone (GH) replacement therapy in adult GH-deficient men. Clinical Endocrinology (Oxford) 2005;63:310–316. doi: 10.1111/j.1365-2265.2005.02343.x. [DOI] [PubMed] [Google Scholar]
- 21.Rahim A, Holmes SJ, Adams JE, et al. Long-term change in the bone mineral density of adults with adult onset growth hormone (GH) deficiency in response to short or long-term GH replacement therapy. Clinical Endocrinology (Oxford) 1998;48:463–469. doi: 10.1046/j.1365-2265.1998.00465.x. [DOI] [PubMed] [Google Scholar]
- 22.Hazem A, Elamin MB, Bancos I, et al. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. European Journal of Endocrinology. 2012;166:13–20. doi: 10.1530/EJE-11-0558. [DOI] [PubMed] [Google Scholar]
- 23.Bachrach LK, Hastie T, Wang MC, et al. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. Journal of Clinical Endocrinology and Metabolism. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 24.Baroncelli GI, Bertelloni S, Sodini F, et al. Acquisition of bone mass in normal individuals and in patients with growth hormone deficiency. Journal of Pediatric Endocrinology and Metabolism. 2003;16(Suppl 2):327–335. [PubMed] [Google Scholar]
- 25.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 26.Bex M, Abs R, Maiter D, et al. The effects of growth hormone replacement therapy on bone metabolism in adultonset growth hormone deficiency: a 2-year open randomized controlled multicenter trial. Journal of Bone and Mineral Research. 2002;17:1081–1094. doi: 10.1359/jbmr.2002.17.6.1081. [DOI] [PubMed] [Google Scholar]
- 27.Bex M, Bouillon R. Growth hormone and bone health. Hormone Research. 2003;60(Suppl 3):80–86. doi: 10.1159/000074507. [DOI] [PubMed] [Google Scholar]
- 28.Bravenboer N, Holzmann PJ, ter Maaten JC, et al. Effect of long-term growth hormone treatment on bone mass and bone metabolism in growth hormone-deficient men. Journal of Bone and Mineral Research. 2005;20:1778–1784. doi: 10.1359/JBMR.050613. [DOI] [PubMed] [Google Scholar]
- 29.Joseph F, Ahmad AM, Ul-Haq M, et al. Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. Journal of Bone and Mineral Research. 2008;23:721–729. doi: 10.1359/jbmr.071117. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto T, Kaji H, Nakaoka D, et al. Effect of lowdose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis. European Journal of Endocrinology. 2002;147:339–348. doi: 10.1530/eje.0.1470339. [DOI] [PubMed] [Google Scholar]
- 31.Tritos NA, Biller BM. Growth hormone and bone. Current Opinion in Endocrinology, Diabetes, and Obesity. 2009;16:415–422. doi: 10.1097/MED.0b013e3283319e6d. [DOI] [PubMed] [Google Scholar]
- 32.Cook DM. Growth hormone and estrogen: a clinician’s approach. Journal of Pediatric Endocrinology and Metabolism. 2004;17(Suppl 4):1273–1276. [PubMed] [Google Scholar]
- 33.Cook DM, Ludlam WH, Cook MB. Route of estrogen administration helps to determine growth hormone (GH) replacement dose in GH-deficient adults. Journal of Clinical Endocrinology and Metabolism. 1999;84:3956–3960. doi: 10.1210/jcem.84.11.6113. [DOI] [PubMed] [Google Scholar]
- 34.Abs R, Feldt-Rasmussen U, editors. Growth Hormone Deficiency in Adults: 10 Years of KIMS. Oxford PharmaGenesisTM Ltd; Oxford: 2004. The KIMS 2004 Aetiology of Growth Hormone Deficiency (KIMS Classification List) pp. 346–348. [Google Scholar]