Abstract
Since the kidney is integral to maintenance of fluid and ion homeostasis, and therefore blood pressure regulation, its proper function is paramount. Circadian fluctuations in blood pressure, renal blood flow, glomerular filtration rate, and sodium and water excretion have been documented for decades, if not longer. Recent studies on the role of circadian clock proteins in the regulation of a variety of renal transport genes suggest that the molecular clock in the kidney controls circadian fluctuations in renal function. The circadian clock appears to be a critical regulator of renal function with important implications for the treatment of renal pathologies which include chronic kidney disease and hypertension. The development, regulation, and mechanism of the kidney clock are reviewed here.
Keywords: kidney, ion transport, electrolyte, clock, hypertension, sodium, potassium
Introduction
Many aspects of behavior and physiology cycle over the course of ~24 hours, being synchronized to the day/night cycle, and are accordingly called circadian (latin for “around a day”) rhythms. Circadian rhythms in physiological function are critical to maintaining health and the biochemical pathways mediating these effects are highly conserved and are present in species from archaebacteria to humans (reviewed in (Richards and Gumz 2012)). The pacemaker of the circadian clock is located in the suprachiasmatic nucleus (SCN) of the brain and is entrained by light (Dibner et al. 2010; Partch et al. 2014). For this central clock, light is a dominant Zeitgeber, literally translated as “time giver,” a signal for entrainment. The central clock synchronizes the peripheral clocks, located in other areas of the brain and in tissues throughout the body, via neuronal and humoral signaling. While light is the dominant Zeitgeber for central clock entrainment, metabolic cues likely act as an additional Zeitgeber for peripheral clocks such as those located in the liver and kidneys.
While the circadian clock is composed of many genes, the core molecular clock mechanism is comprised of four key circadian genes: Clock, Bmal1, Period (Per homologs 1, 2 and 3) and Cryptochrome (Cry homologs 1 and 2). These genes encode proteins that function in a feedback loop to regulate transcription of clock-controlled genes (for an excellent review on this mechanism, see (Partch et al. 2014)). Briefly, circadian proteins CLOCK and BMAL1 form a heterodimer which binds to E-box elements of clock-controlled genes. This BMAL1/CLOCK heterodimer also binds to E-box elements in the promoters of the Cry and Per genes to activate transcription, thus forming the positive arm of the feedback loop. In the negative arm of the loop, PER and CRY then act on BMAL1/CLOCK to inhibit their own transcription. Two nuclear receptors involved in the molecular clock regulatory loops are the retinoid-related orphan receptor (ROR) and REV-ERBα. ROR acts positively on Bmal1 whereas REV-ERBα mediates opposing action on Bmal1. Kinase and phosphatase-mediated post-translational modifications provide an additional layer of regulation for clock proteins. For example, nuclear entry of PER and CRY is regulated via phosphorylation by Casein Kinase 1 isoforms δ/ε (CK1δ/ε) (Badura et al. 2007) (Lee et al. 2011). The molecular circadian clock mediates the regulation of rhythmic physiological function via transcriptional control and post-transcriptional control of downstream clock target genes and this regulation occurs in a tissue-specific manner. In an elegant genome-wide study in the liver, Koike et al. demonstrated that the core circadian clock proteins interact with regulatory sequence elements in several thousand genes (Koike et al. 2012). Studies such as these are increasing our understanding of circadian clock function and shedding light on the tissue-specific effects of the clock. A recent landmark genomics study characterized circadian gene expression over a 48 hour period in wild-type mice in a variety of tissues, including the kidney (Zhang et al. 2014). This work demonstrated that more than 40% of all expressed genes in the tissues tested exhibited circadian variations in expression. In terms of the absolute numbers of cycling genes, the kidney was second only to the liver with several thousand genes exhibiting circadian expression.
In his 1985 Bowditch Lecture, Moore-Ede discussed the concept of physiological homeostasis, or equilibrium (Moore-Ede 1986). In the 19th century, Claude Bernard popularized the notion that the body is in a constant, fixed state. It wasn’t until 1925 that Cannon and other physiologists developed the concept of homeostasis: that the body makes constant changes to maintain a steady state. It was thought that the body reacted to daily activities in order to maintain homeostasis. For instance, in response to sodium intake, the body would then react to excrete excess sodium from the body. In his lecture, Moore-Ede talked about predictive homeostasis in which the body starts to enact changes to counter activities it can anticipate because they occur in a predictable, cyclical manner. Thus, if meals are frequently eaten at the same time each day, the body can start to prepare for this predicted meal before it occurs by inducing expression of necessary solute transporters and metabolic enzymes in the liver, gut and kidney, for example. Discovery of the molecular clock components provided evidence that this predictive homeostasis occurs due to the activity of the circadian clock; the clock allows the body to keep track of the time using multiple cues such as light and food intake. While laying down at night suppresses urinary sodium, potassium and water excretion, excretion is not suppressed by laying down during the day (Ede et al. 1972). The body predicts we are going to bed at night and prevents loss of precious nutrients. The kidney provides a textbook illustration of predictive homeostasis. Here, we review the evidence for circadian control of rhythms in renal function and present the current state of knowledge regarding the regulation and function of the clock in the kidney.
The Kidney
The kidneys function to filter the blood, maintaining fluid and ion homeostasis (Palmer and Schnermann 2015). This is crucial and tightly regulated because even slight changes in filtrate reabsorption can lead to large changes in blood chemistry and blood pressure. Sustained hypertension (high blood pressure) can lead to a number of diseases and is a main risk factor for chronic kidney disease (CKD) and cardiovascular disease, the main cause of death for about 1/3 of Americans ever year (Go et al. 2014). The kidney achieves this fine level of regulation via the many specialized cell types located along the segmented nephron, the functional unit of the kidney (Figure 1). An adult kidney contains about 1 million nephrons on average and each one is tightly controlled to avoid large fluctuations in blood pressure. Blood enters every nephron and is filtered through the glomerulus. The filtrate flows through the nephron and the many specialized cell types in each segment of the nephron reabsorb or secrete solutes according to the needs of the body. The final filtrate flows into the ureter to eventually become the urine.
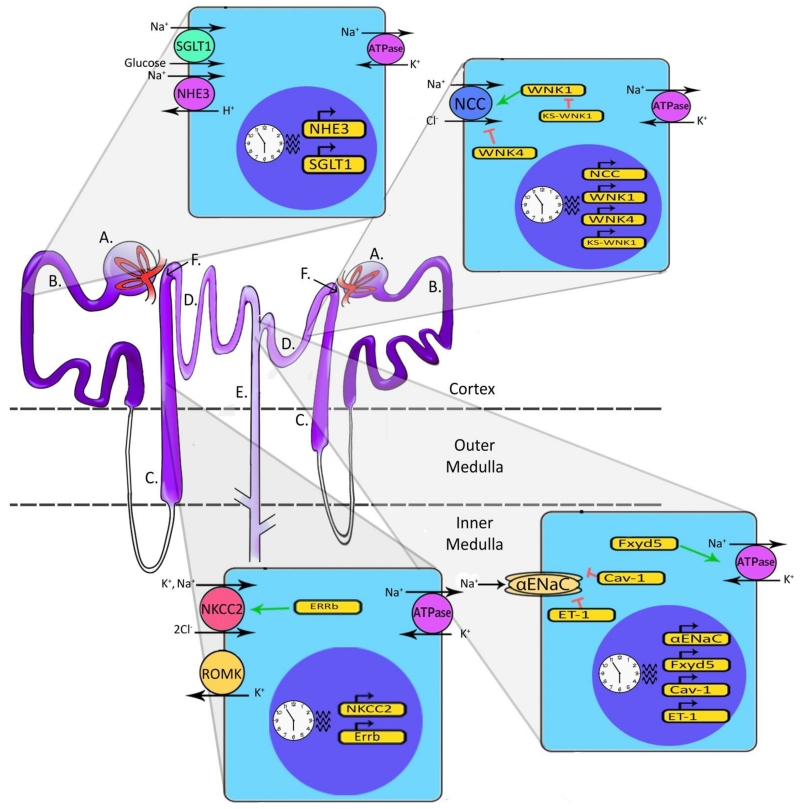
Figure 1. Diagram of Two Nephrons and Representative Cell Types.
A. Blood enters the nephron and is filtered at the glomerulus. B. Filtrate then moves to the Proximal Tubule (PT). This segment of the nephron contains the sodium-glucose linked transporter isoform 1 (SGLT1) and the sodium-hydrogen exchanger isoform 3 (NHE3), both of which are located on the apical membrane (facing the filtrate) and are regulated by the circadian clock. Sodium reabsorbed from the filtrate is pumped back into the blood by the basolateral Na/K-ATPase, found on each of the cell types illustrated here. C. Filtrate then moves to the Loop of Henle. Cells in the Thick Ascending Loop (TAL) contain the sodium-potassium-chloride cotransporter isoform 2 (NKCC2) which is regulated by the nuclear receptor ERRβ. These cells also contain renal outer medullary potassium channel (ROMK), responsible for pumping K+ back into the filtrate. Again, these cells have a basolateral Na, K-ATPase. D. The distal convoluted tubule (DCT) cells contain apically located sodium chloride cotransporters (NCC) which reabsorb Na+ and Cl− from the filtrate. This co-transporter is regulated by the circadian clock and well as with-no-lysine (WNK) kinases. E. The last part of the nephron filtrate enters is the Collecting Duct (CD). The epithelial sodium channel (ENaC) reabsorbs Na+ from the filtrate and is regulated by the clock. FXYD5, CAV-1 and ET-1 are regulated by the circadian clock as well and in turn FXYD5 positively regulates the Na/K-ATPase, while CAV-1 negatively regulates ENaC. F. Juxtaglomerular apparatus: Cells in the macula densa communicate with the glomerulus to modulate GFR in a process known as tubule-glomerular feedback (TGF). Glomeruli, PTs and DCTs are located in the cortex of the kidney while the Loop of Henle and part of the collecting duct span into the medulla. Multiple collecting ducts converge to ultimately transport urine out of the kidney.
The kidney carefully maintains homeostasis for sodium, potassium and several other key solutes in addition to maintaining acid/base balance. Each segment of the nephron has specific channels and transporters dedicated to the transport of sodium, potassium, hydrogen and bicarbonate, in addition to other important ions and solutes. Each is regulated differently, allowing for fine-tuned control of each segment in order to maintain homeostasis. Because much of the work on the circadian clock in the kidney has focused on sodium balance and effects on blood pressure, this review will focus on regulation of renal sodium handling by the molecular clock.
After passing through the glomerulus, the filtrate from the blood enters the proximal tubule (PT) where about 60% of sodium reabsorption takes place (reviewed in (Palmer and Schnermann 2015)). Cells in this segment of the nephron express an apical (facing the lumen of the tubule) sodium-hydrogen exchanger (NHE3) and sodium glucose transporter (SGLT1); expression of these transporters appears to be regulated by the circadian clock (Saifur Rohman et al. 2005)(CircaDB (Pizarro et al. 2013) http://circadb.hogeneschlab.org/). Filtrate then moves to the Loop of Henle where around 25% of sodium reabsorption occurs in the thick ascending portion (TAL). In this segment, the clock appears to regulate expression of the sodium-potassium-2 chloride co-transporter (NKCC2) and the estrogen-related receptor beta (ERRβ) (CircaDB (Pizarro et al. 2013))(Krid et al. 2012). The next segment, the distal convoluted tubule (DCT), accounts for about 10% of sodium reabsorption and this occurs mainly through the sodium chloride co-transporter (NCC). NCC is regulated by a kinase cascade involving members of the with-no-lysine (WNK) family. Increasing evidence suggests that NCC and the WNK pathway are subject to regulation by the circadian clock (Richards et al. 2014a; Susa et al. 2012). The final segment the filtrate enters is the collecting duct (CD) where only about 5% of sodium reabsorption occurs. However, the distal nephron is the most highly regulated segment of the nephron. In addition to transporters and exchangers, ion channels are also integral to sodium reabsorption. In principal cells of the CD, sodium entry occurs through the apical epithelial sodium channel (ENaC). The alpha subunit of ENaC (αENaC) and other key regulators of sodium transport in this segment have all been linked to the clock (Zuber et al. 2009) (Gumz et al. 2009; Stow et al. 2012).
Early evidence for the existence of a “kidney clock” comes from a 1952 study of five human subjects in which activity, light, temperature, diet and other variables which may affect circadian rhythms were carefully controlled and monitored. Rhythms in urine flow and pH were observed in addition to sodium (Na+) and potassium output (K+), both peaking shortly after waking (Mills and Stanbury 1952). In 1975, the circadian rhythm of potassium excretion was observed in three males who maintained a constant supine position and subsisted on a liquid diet administered every three hours (Moore Ede et al. 1975). These studies provided the first evidence for a kidney-specific clock.
Subsequent work continued to demonstrate that renal function appears to vary with a circadian rhythm. For example, many aspects of kidney function exhibit circadian fluctuations, such as glomerular filtration rate (GFR) (Koopman et al. 1989), sodium excretion and renal blood flow (Pons et al. 1996). At a transcriptional level, expression of clock genes including Clock, Bmal1, Cry1, Cry2, Per1 and Per2 oscillate with a ~24 hour rhythm (Reppert and Weaver 2002). Urinary sodium excretion exhibits a circadian pattern in rodents, primates and humans (reviewed in (Stow and Gumz 2011)) and this may be explained by the clock-mediated regulation of a number of renal sodium transport genes (Zuber et al. 2009) (Gumz et al. 2009; Saifur Rohman et al. 2005). As illustrated in Figure 1, circadian clock-mediated regulation of a several key transport genes in the kidney has been established. In the following sections, the evidence for this regulation is considered in detail.
Hormones and the kidney clock
The kidney is both an important target and a source of hormones critical for maintaining ion homeostasis and blood pressure control. The circadian rhythm of plasma sodium is in phase with many hormones involved in blood pressure homeostasis including angiotensin II, aldosterone (Hilfenhaus 1976) (Jensen and Pedersen 1997) and vasopressin (Morawska-Barszczewska et al. 1996) as well as angiotensin converting enzyme (ACE) (Stepien et al. 1993) and plasma renin activity (Hilfenhaus 1976) (Jensen and Pedersen 1997), detailed below. Thus, proper regulation of these hormones is critical for blood pressure homeostasis.
All forms of Mendelian (inherited) hypertension are due to a defect in the Renin-Angiotensin-Aldosterone System (RAAS) (Lifton et al. 2001). The RAAS acts to maintain blood pressure homeostasis. When low blood pressure is sensed, juxtaglomerular cells in the nephron release the enzyme renin. Renin then converts angiotensinogen to angiotensin I. Angiotensin I then is further converted to Angiotensin II (Ang II) by ACE. Ang II acts on the vessels as a vasoconstrictor but can also stimulate the adrenal glands to secrete aldosterone. Aldosterone further stimulates sodium transporters in the aldosterone-sensitive distal nephron, leading to an increase in sodium reabsorption and blood pressure. An intrarenal RAAS exists in addition to the well-characterized systemic RAAS (Kobori et al. 2007; Moon 2013; Prieto et al. 2013), further solidifying a role for the in the kidneys in the development of high blood pressure.
In a human study, Ang II levels were examined from 15 patients around 14 years of age who had monosymptomatic nocturnal enuresis (MNE) and 10 normal patients of a similar age (Rittig et al. 2006). They found that within the MNE group, Ang II was expressed in a time-dependent manner with nighttime levels being more than twice as high as daytime levels in patients without polyuria (excess urination). The MNE group with polyuria did not show any significant variation in Ang II levels between day and night, suggesting that disrupted circadian rhythms of Ang II may play a part in the pathogenesis of MNE.
The peptide hormone atrial natriuretic peptide (ANP) is made in the heart and is a vasodilator. Its actions on the kidney can lead to water and electrolyte excretion which, combined with vasodilatory properties, lead to an overall decrease in blood pressure. Reports of daily, rhythmic changes in levels of this peptide in humans are conflicting and often have small subject numbers. In one study, ANP levels in humans (10 normotensive patients and 10 hypertensive patients), display a rhythm that appears to be antiphase to blood pressure and HR rhythms (Portaluppi et al. 1990). Furthermore, in normotensive subjects, there was a correlation between ANP, renin and aldosterone levels while this was not observed in the hypertensive group. In another study of 12 males, the authors state no rhythm of ANP but a 15% increase at night (Kool et al. 1994). In 21 healthy, middle aged men, ANP has a significant rhythmic expression, with the peak of expression occurring at night (Vesely et al. 1996). These results are similar to those obtained for 20 year old men and women (Winters et al. 1988). While it is definitely possible that daily variation of ANP can vary between humans and other animals, more studies would be helpful to confirm these findings.
Urodilatin is encoded by the same gene as ANP but has four extra amino acids on its N-terminus (Vesely 2007). Unlike ANP, urodilatin is only secreted in the kidney and is not secreted into the systemic circulation. It causes inhibition of sodium and water reabsorption when high blood pressure is sensed. In 1991, ten years after ANP was discovered, Drummer et al. described the time-dependent rhythm of urodilatin (Drummer et al. 1991). In six healthy men, a rhythm of urodilatin was shown with a maximal expression during the day. However, in this study, a significant rhythm for ANP immunoreactivity was not observed.
Dopamine is released from nerves in the kidney and it is synthesized by cells in the proximal tubule (reviewed in (Choi et al. 2015)). In the kidney, dopamine inhibits sodium and water reabsorption when extracelluar fluid (ECF) volume is increased. Under constant light and under normal 12:12 LD, there were time-dependent changes in dopamine expression in the striatum and nucleus accumbens of rats (Castaneda et al. 2004). There was no circadian rhythm of dopamine expression under constant darkness, however, demonstration that variations in dopamine levels are likely dependent on time cues. While the circadian expression of dopamine in the kidney has not yet been investigated, it does appear to have a circadian rhythm in mouse adrenal glands and skeletal muscle (CircaDB (Pizarro et al. 2013)). It would be interesting to investigate the expression of dopamine in the kidney as well, given that it regulates water and sodium handling and in turn, blood pressure.
Vasopressin, also called antidiuretic hormone (ADH), is released when there is a decrease in extracellular fluid volume. It causes an increase in water reabsorption in the collecting duct, thus decreasing diuresis as the name implies. Challet et al. looked at the rhythm of vasopressin in mice lacking folate, vitamin B9 (Challet et al. 2013). It has previously been shown that folate may be a cofactor for cryptochromes (Ozgur and Sancar 2003). It has also been shown that rats lacking folate have decreased amplitude of melatonin secretion (Fournier et al. 2002), which is important for maintaining the sleep-wake cycle. This group found that their folate-deficient mice had decreased rhythms of vasopressin expression compared to normal mice (Challet et al. 2013). The authors further point out that folate reduction is commonly seen with aging. Decrease or loss of circadian rhythms has also been associated with increasing age (Weinert 2000), although whether it is a cause or result of the aging process is unclear. It also leads to the possibility that these disruptions in circadian rhythms may be implicated in pathogenesis of many diseases associated with aging.
The kidney is mainly responsible for excretion of cortisol and its metabolites and this process is impaired in chronic kidney disease patients (Walker et al. 1992) and reviewed in (Bonny et al. 2013). In healthy subjects, aldosterone and cortisol exhibit time-dependent increases in the early morning. In anephric patients, the correlation between plasma aldosterone and plasma cortisol is lost: daily variation in plasma aldosterone is not present whereas plasma cortisol rhythms remain (Cooke et al. 1979). Rhythmic changes in urinary cortisol and aldosterone excretion are disrupted in kidney transplant patients as well (Stefanovic et al. 1995). Renal handling of cortisol and aldosterone may affect adrenal hormone production through disruption of known negative feedback loops. For example, synthesis of the pituitary hormone adrenocorticotropic hormone (ACTH), which stimulates cortisol production by the adrenals, is inhibited by high levels of cortisol in the circulation. Thus, decreased renal function, or perhaps, disruption of the kidney clock, negatively affects cortisol handling and may subsequently alter glucocorticoid production inappropriately. Although central and peripheral circadian clocks certainly contribute to regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Kino 2012) (Leliavski et al. 2015), the role of the molecular kidney clock in these processes remains poorly understood.
The Kidney Clock
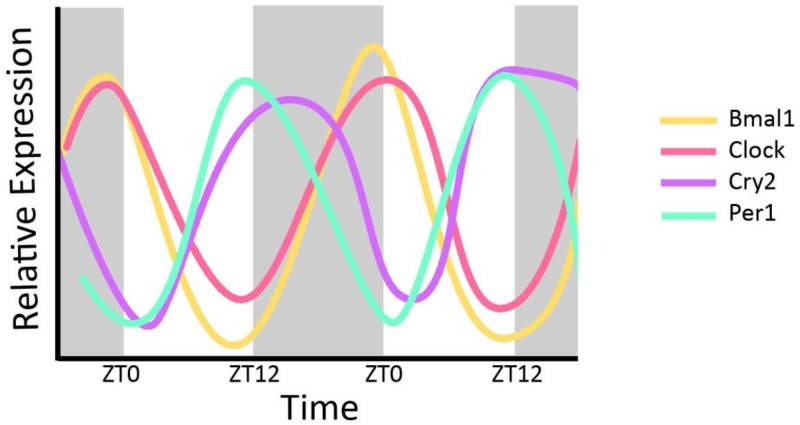
The core circadian clock proteins regulate almost half of all expressed genes and do so in a tissue-specific manner (Zhang et al. 2014). As illustrated in Figure 2, Bmal1, Clock, Per1, and Cry2 exhibit clear circadian variation in expression in the whole kidney over a 48 hr period (CircaDB (Pizarro et al. 2013)). Rodent models have proven invaluable to our understanding of the mechanism of the renal circadian clock and its impact on blood pressure regulation. An animal model of CKD is produced by removing one entire kidney and 2/3 of the other kidney, removing about 5/6 of the total kidney mass and hence providing the name for this model, the 5/6 nephrectomy. In mice, this causes an increase in MAP of about 30 mmHg two weeks after the procedure (Gava et al. 2012). In rats, this caused peak Bmal1 expression in the remnant kidney to shift 4 hours earlier than sham operated rats (Huang et al. 2013). Using immunohistochemistry to determine localization of protein, DBP localization changed from mainly glomerular in the sham controls to much more widely dispersed in the CKD model rats. PER2 protein expression was high in the corticomedullary junction in controls and decreased in CKD rats. Conversely, expression was highly increased in the cortex of CKD rats compared to controls. Thus, not only can circadian proteins mediate changes in blood pressure, changes in blood pressure can affect localization of circadian proteins in the kidney.
Figure 2. Circadian Expression of Core Clock Genes in the Kidney.
Relative expression levels are plotted on the y-axis with Zeitgeber time on the x-axis. Shaded areas represent time when lights are off. Peak Expression of Bmal1 and Clock are in phase and approximately antiphase to Cry2 and Per1 mRNA expression. Data derived from CircaDB (Pizarro et al. 2013).
The link between the kidney and maintenance of normal blood pressure was further illustrated using spontaneously hypertensive rats (SHRs). Kidneys transplanted from normotensive rats into SHRs lead to a decrease in mean arterial pressure of over 50 mmHg compared to sham-operated controls (Grisk et al. 2002). Conversely, when kidneys from SHRs were transplanted into normotensive rats, the MAP increased by around 40 mmHg. The fact that a hypertensive phenotype tracks with the kidney to such a large degree highlights the magnitude of the renal contribution to blood pressure homeostasis. While the SHRs display daily rhythms in plasma sodium concentration (Fang et al. 2000), closer investigation into the contribution of the kidney clock to this phenotype remains to be done.
Animal models with disruption of circadian genes of interest also provide evidence for the roles of specific clock genes. However, it is important to keep in mind that most of these models have disruption of clock genes systemically, so while the phenotypes observed certainly support heavy renal involvement, participation from other systems cannot be ruled out. Global knockout (KO) of Bmal1 results in complete loss of circadian rhythmicity measured via wheel running activity (Bunger et al. 2000). These mice are also sterile (Alvarez et al. 2008) and have shorter lifespans than wild type mice (Sun et al. 2006). They have a loss of circadian rhythmicity in HR and blood pressure and are also hypotensive compared to the wild type mice. (Curtis et al. 2007). Whether a renal phenotype exists in global Bmal1 KO mice has yet to be determined.
The circadian proteins are important for blood pressure control through mechanisms involving multiple tissues. Loss of Bmal1 specifically in the forebrain leads to mice with a loss of a circadian pattern of activity in constant light or constant dark conditions but normal rhythms in LD (12 hours light, 12 hours dark) and otherwise healthy, fertile mice (Izumo et al. 2014).
In the first cell-type specific KO of a clock gene in the kidney, Firsov and colleagues generated mice lacking BMAL1 in renin-producing cells (Tokonami et al. 2014). Expression of BMAL1 was lost in cells of the juxtaglomerular apparatus (see Figure 1F) and in some cells of the CD. These kidney-specific Bmal1 KO mice have decreased plasma aldosterone compared to control mice and also have significantly lower blood pressure compared to controls. Thus, the loss of BMAL1 only in certain cells of the kidney was profound enough to cause systemic changes in aldosterone and blood pressure.
Clock KO mice are hypotensive as well, yet they retain the normal 24 hour rhythmic variation in blood pressure (Zuber et al. 2009). Expression of 20-HETE (20-hydroxyeicosatetraenoic acid), which is a regulator of blood pressure, is also altered in these mice (Nikolaeva et al. 2012). If acting on preglomerular arterioles in the kidney, 20-HETE leads to an increase in blood pressure through constriction of the arterioles (Nikolaeva et al. 2012). However, 20-HETE also inhibits some of the sodium transporters found in the PT and TAL of the nephron which leads to less sodium reabsorption and a consequent decrease in blood pressure. Therefore, the dysregulation of 20-HETE has been proposed as a contributory mechanism to the hypotensive phenotype shown in Clock KO mice. While these mice have similar mean levels of plasma aldosterone compared to WTs, they have significantly lower levels at ZT12 (Nikolaeva et al. 2012), which stands for Zeitgeber Time 12 – 12 hours after the lights have been turned on and on a 12:12 LD cycle, the onset of darkness. Interestingly, ZT12 is the time that Per1 mRNA expression peaks in mouse kidney (CircaDB (Pizarro et al. 2013)).
In 2005, the first evidence for circadian control of a renal gene was provided by Okamura and colleagues (Saifur Rohman et al. 2005). They showed that NHE3 (encoded by the gene Slc9a3) mRNA expression is circadian and furthermore is regulated by CLOCK:BMAL1 heterodimer binding to an E-box element on Slc9a3. The circadian expression of NHE3 mRNA is severely blunted in Cry1/2 KO mice (Saifur Rohman et al. 2005), further supporting the circadian control of this gene. These mice also exhibit salt-sensitive hypertension due to increased levels of aldosterone (Doi et al. 2010). Sglt1 (encoded by Slc5a1) and its expression is also circadian: SGLT1 mRNA expression peaks around ZT12 in the mouse kidney while NHE3 mRNA expression peaks about two hours earlier (data derived from CircaDB, see (Pizarro et al. 2013)).
Dbp/Hlf/Tef triple KO mice lack three circadian clock regulated genes: D-site-binding protein (DBP), hepatic leukemia factor (HLF), and thyrotroph embryonic factor (TEF) (Gachon et al. 2006). Mice lacking one or two of these factors have mild phenotypes which may be due to the fact that DBP, TEF and HLF have well conserved amino acid sequences and thus they likely compensate for each other in the single or double KO mice. Mice lacking all three usually do not live longer than 1 year, with symptoms such as seizures occurring during the first 3 months of life (Gachon et al. 2004). In the kidney of the triple KO mice, Northern blot analysis revealed significantly less mRNA from potential target genes involved in detoxification and drug metabolism. This may be related to the mechanism of variable drug effectiveness depending on the time of administration. Later, this group showed that the triple KOs had low blood pressure, decreased aldosterone levels and cardiac hypertrophy (Wang et al. 2010).
Another useful model of circadian gene disruption is the PER2 mutant mouse (Vukolic et al. 2010). The blunted PER2 protein expressed in these mice is missing dimerization sites (PAS B and PAC domains) (Zheng et al. 1999). These domains have been implicated in the ability of PER2 to interact with other proteins and possibly act as a light sensor through FAD (Flavin Adenine Dinucleotide) binding (Bibikov et al. 1997) (Hill et al. 1996). Indeed, mice with mutant Per2 (and also Per1) genes have altered responses to light (Albrecht et al. 2001). This group found that the mutant mice have higher heart rate during the light period than wild type mice but have similar locomotor activity. In normal LD conditions, PER2 mutant mice had circadian periods of mean arterial pressure (MAP), heart rate (HR) and activity similar to WT mice. However, in the absence of light (DD), the mutant mice had shorter periods with a trend of decreased amplitude of MAP, HR and activity compared to WT controls. The mutant mice also were unable to maintain day-night differences in MAP, HR and activity when put into constant dark conditions for 8 days, but showed improved differences when returned to LD.
In addition to CRY and PER, DEC1 and DEC2 are also involved in the negative arm of the transcriptional circadian feedback loops (Honma et al. 2002). Gene expression of both Dec1 and Dec2 exhibits a circadian pattern of expression in the rat heart while only Dec2 shows a comparable rhythm in the kidney (Wu et al. 2011). Since rats are nocturnal, they normally eat at night. When food intake was restricted to the light cycle/rest phase for seven days, Dec1 and Dec2 mRNA expression in the heart was shifted by about 8 hours. In the kidney, however, Dec1 expression became rhythmic and the peak of Dec2 expression was shifted 4 hours earlier than controls. The authors also reversed both feeding time and the LD schedule. After 7 days, the peak of Dec1 expression was shifted only in the heart while the peak of Dec2 expression was shifted in both the heart and kidney. This provides evidence that the timing of food cues can actually induce rhythmic expression of clock-controlled genes in the rat kidney.
Contrary to the rats from the previous experiments, Dec1 exhibits a circadian rhythm of expression in the mouse kidney and liver, and this rhythm is disrupted in mice expressing a mutant CLOCK protein resulting from deletion of exon 19 (Noshiro et al. 2005). In the kidney, mutant CLOCK expression was associated with decreased total Dec1 expression. However, rhythmic expression of Dec1 mRNA was not affected in LD or DD, suggesting that loss of wild type CLOCK expression was not sufficient to alter circadian expression. Loss of DEC1 does not appear to negatively affect mice compared to WT controls (Sun et al. 2001). Since DEC1 and DEC2 have similar DNA binding domains, it seems that DEC1 may be redundant in the kidney with DEC2 able to compensate for loss of DEC1. This possible redundancy suggests the importance of maintaining the circadian clockwork in the kidney.
PER1 action in the kidney
Due to the important role that ENaC plays in maintaining sodium balance, it is tightly regulated at multiple levels. PER1 has been a focus of investigation in the kidney because it is a direct target gene of the sodium- and blood pressure-regulating hormone aldosterone (Gumz et al. 2003) which regulates ENaC via multiple mechanisms (Rossier 2014). Aldosterone-mediated transcriptional regulation of the Scnn1a gene, encoding αENaC, occurs through the mineralocorticoid receptor (MR). PER1 appears to be involved in this regulation (Richards et al. 2013d). In one of the first reports to link the molecular clock to renal sodium handling, Gumz and colleagues demonstrated that knockdown of PER1 in several renal CD cell lines resulted in decreased expression of αENaC (Gumz et al. 2009). Furthermore, Per1 KO mice exhibited increased urinary sodium excretion, again supporting a role for the molecular clock in the regulation of renal sodium handling. In order for PER1 to get into the nucleus and affect its target genes, it must be phosphorylated by CK1δ/ε. A CK1δ/ε inhibitor (PF670462) prevents PER1 nuclear entry in a murine cell line model of the CCD, mpkCCDc14 cells (Richards et al. 2012). If PER1 is unable to enter the nucleus, it is therefore unable to associate with E-box elements of target genes such as αENaC. Inhibition of PER1 nuclear entry decreases baseline αENaC mRNA expression, suggesting that PER1 mediates basal expression of αENaC as well as increasing transcription. αENaC protein levels in the membrane are also decreased by about 60% after CK1δ/ε blockade.
In addition to regulating αENaC, PER1 regulates several other genes in the kidney (Stow et al. 2012). Per1 levels were decreased using siRNA in mpkCCDc14 cells which led to a decrease in mRNA expression of Fxyd5. Conversely, Ubiquitin-Conjugating Enzyme E2E3 (Ube2e3), Caveolin-1 (Cav-1) and Endothelin-1 (ET-1, encoded by Edn1) expression increased following PER1 knockdown. FXYD5 increases activity of the Na, K-ATPase which is responsible for pumping sodium reabsorbed from the filtrate back in to the blood (Lubarski et al. 2005).
UBE2E3 is an E3 ubiquitin ligase, an enzyme which adds ubiquitin molecules to proteins, a common signal for proteasomal degradation. When UBE2E3 ubiquitinylates ENaC, it is removed from the membrane, thus decreasing sodium reabsorption (Debonneville and Staub 2004). Reduction of PER1 levels lead to increased expression of this ligase, potentially leading to increased ENaC degradation. CAV-1 is a lipid raft protein associated with removal of ENaC from the membrane as well (Lee et al. 2009), leading to decreases in sodium reabsorption like UBE2E3. Finally, ET-1 leads to decreased ENaC open probability through a mechanism involving nitric oxide and the Endothelin type B (ETB) receptor (Bugaj et al. 2008; Gallego and Ling 1996). Following PER1 knockdown, ET-1 expression increased almost four-fold (Stow et al. 2012). PER1 knockdown lead to decreased membrane αENaC protein levels, potentially due to contribution of the increased amount of ET-1, CAV-1 and UBE2E3 (Richards et al. 2012).
Per1 KO mice on a 129/sv background exhibit blood pressures about 18 mmHg lower than wild type mice but maintain normal blood pressure rhythms (Stow et al. 2012). Although the extra-renal contributions to this phenotype have not been evaluated, many pieces of evidence support role of the renal clock. Per1 KO mice also have decreased basal levels of αENaC mRNA in the medulla compared to WT mice (Gumz et al. 2009). These mice exhibit increased levels of ET-1 in the cortex and medulla of the kidney. While known as a potent vasoconstrictor in the majority of the body, ET-1 acts to decrease blood pressure and increase natriuresis (sodium excretion) in the kidney through inhibition of ENaC (Lynch et al. 2013) (Bugaj et al. 2008). Furthermore, ET-1 peptide levels are increased in ex vivo IMCD cells from Per1 heterozygous mice (which have approximately 50% less PER1 expression than WT mice) (Richards et al. 2014b). Increased ENaC inhibition by ET-1 coupled with decreased basal ENaC levels may contribute to the low blood pressure phenotype observed in the global Per1 KO mice. In healthy humans, plasma ET-1 levels decrease at night when blood pressure dips, whereas patients with CKD have increased night time plasma ET-1 levels that correlate with loss of night time blood pressure dipping (Dhaun et al. 2014). Normally, people experience a 10-20% decrease in blood pressure at night and those who don’t are called non-dippers. Non-dipping has been linked to increased risk of not only cardiovascular events (Ohkubo et al. 2002) but also chronic kidney disease (Hermida et al. 2010; Kario and Shimada 2004).Together these results suggest that ET-1 may contribute to the circadian profile of blood pressure.
Per1 heterozygous mice also have decreased levels of plasma aldosterone relative to wild type 129/sv mice (Richards et al. 2013b). Aldosterone regulation of ENaC expression is mediated at least in part by PER1. Treating mpkCCDc14 cells with aldosterone leads to increased interaction of PER1 with E-box elements in the αENaC promoter (Richards et al. 2013d). Increased binding of RNA POLII to the ENaC promoter in the presence of aldosterone was observed as well, consistent with the known effect of aldosterone to increase αENaC transcription. This is further validated by the observation that PER1 knockdown in the presence of aldosterone is associated with decreased αENaC expression compared to aldosterone alone (Gumz et al. 2009). Similar results were observed following nuclear blockade of PER1 using a CK1δ/ε inhibitor (Richards et al. 2012).
In addition to PER1 regulation by aldosterone, it seems that aldosterone may be regulated by PER1. In addition to lower plasma aldosterone levels, Per1 heterozygous mice do not exhibit the normal increase in plasma aldosterone during their active phase that occurs in wild type mice (Richards et al. 2013a). One explanation for this phenotype may be that Per1 heterozygous mice have a decrease in 3β-HSD (3-(β)-hydroxysteroid dehydrogenase) expression. 3β-HSD is an enzyme produced in zona glomerulosa cells of the adrenal glands, which are responsible for the production of aldosterone, among other hormones. This enzyme catalyzes the synthesis of progesterone, which is a precursor to aldosterone in the steroid hormone biosynthesis pathway (Simard et al. 2005). The time-dependent increase in 3β-HSD during the active phase observed in wild type mice is blunted in Per1 heterozygous mice. PER1 knockdown in a human adrenal cell line (NCI-H295R) causes a 58% decrease in 3β-HSD mRNA levels (Richards et al. 2013c). A similar result was obtained in vivo in the adrenal gland in WT 129/sv mice treated with the CK1δ/ε inhibitor.
Cry1/2 KO mice actually have increased levels of 3β-HSD which correlates with the observed increase in their plasma aldosterone levels (Doi et al. 2010). These mice exhibit salt-sensitive hypertension. Interestingly, this phenotype is nearly opposite that of Per1 KO mice which exhibit reduced blood pressure and that of Per1 heterozygous mice which have decreased adrenal gland expression of 3β-HSD (Richards et al. 2013a; Stow et al. 2012). Evidence suggests that PER1 and CRY1/2 have opposing actions on specific target genes encoding αENaC and FXYD5 in the kidney and PPARα and DEC1 in the liver (Richards et al. 2013a). Decreased expression of PER1 expression in vitro and in vivo in the liver and the kidney was associated with an increase in CRY2 protein levels. This is further supported by the observation that inhibition of PER1 nuclear entry with the CK1δ/ε inhibitor in mouse liver cells (AML12) increases cytosolic and nuclear CRY2 expression (Richards et al. 2013c). Further supporting opposing roles of CRY2 and PER1, mice lacking both Per1 and Cry2 have a normalization of their free running period compared to mice lacking Per1 or Cry2 alone (Oster et al. 2003).
The thiazide-sensitive NCC is also regulated by PER1 (Richards et al. 2014a). Either PER1 knockdown or pharmacological blockade of nuclear entry in a model of the DCT (mDCT15 cells) resulted in decreased NCC expression. Furthermore, nuclear blockade of PER1 via CK1δ/ε inhibition resulted in decreased NCC activity levels in these cells. NCC mRNA levels exhibited time-of-day-dependent changes with higher expression during the mouse active period. Consistent with the in vitro data from mDCT15 cells, NCC expression levels were also decreased in the cortex of Per1 heterozygous mice compared to WTs. Similarly, CK1δ/ε inhibitor treatment of WT mice decreased NCC expression compared to vehicle-treated controls. These data suggest that the circadian clock is critical for regulation of many important aspects of renal sodium reabsorption.
Development
The fetal SCN is synchronized by the mother and after birth, maternal care helps to keep the clock entrained (Ohta et al. 2002). Eventually, the neonatal SCN matures enough to take over its job as the central clock (Ohta et al. 2002). To investigate developmental changes in the circadian clock in several tissues, transgenic rats expressing a PER2-LUCIFERASE fusion protein were utilized (Nishide et al. 2014). These rats are transgenic for the mouse Per2 promoter fused to a destabilized luciferase (luciferase fused with a modified PEST sequence (Ueda et al. 2005)) reporter gene (Per2-dLuc) (He et al. 2007). This allows Per2 promoter activity to be visualized by measuring luminescence. Significant differences were shown in Per2-dLuc activity between developmental stages E20 (embryonic day 20), P5 (postnatal day 5), P19 and adult rats. Per2-dLuc activity varied in total luminescence units as well as amplitude of change over time between the different developmental stages in the SCN, lung, kidney and liver in culture. In addition, other tissues were studied and similar tissue-to-tissue variability in Per2-dLuc expression was found. The peak phase of Per2-dLuc advanced after birth to adulthood by about 12 hours in the kidney and lung but not in the liver and only slightly in the SCN between E20 and adult. The SCN was one of the tissues in which Per2-dLuc activity did not vary or shift as much during development even though other tissues displayed significant changes, supporting the idea that peripheral clocks are not solely entrained by the SCN.
One reason for the differential entrainment of the SCN and peripheral clocks could be due to the fact that between 2 and 3 weeks after birth, pups switch from drinking milk to eating chow (Nishide et al. 2014). The different nutrient composition may give different cues to the pups regarding entrainment. Another explanation is that pups usually feed while mothers rest, so they are eating during the day, or what should be their inactive period. The switch from milk to chow would be accompanied by a reversal of feeding time, resynchronizing the peripheral clocks. The different expression of PER2 may also be because organs are not fully mature at birth. The function changes as the pups age and this may also account for the different expression of PER2 due to changing developmental needs (Yamazaki et al. 2009).
In order to investigate embryonic stages of development more thoroughly, gene expression from embryonic days E10-E19 was evaluated in PER2::LUC mice (Dolatshad et al. 2010). Similar to the Per2-dLuc rats, these mice express a PER2-LUCIFERASE fusion protein. These mice are on a C57BL/6J background and have the luciferase gene added in frame to the end of the Per2 gene (Yoo et al. 2004). Real time PCR measurements of clock gene expression in the liver showed little to no circadian rhythms during this time even though maternal rhythms were robust. However, if these tissues were excised and cultured in vitro, large circadian rhythms in PER2::LUC protein were observed. Although this could be an artifact of cell culture, these results do not rule out the possibility that individual cells have rhythms and they are just not yet synchronized at embryonic stages.
Circadian variation during development of other clock controlled genes and kidney-specific clock controlled genes was studied in the kidneys of Sprague-Dawley rats. While small-amplitude rhythms of expression of Bmal1, Rev-erbα, Per2 and Cry1 mRNA were seen at E20, the rhythms became more robust with increasing peak-to-trough differences from 1 week, 4 weeks and 12 weeks after birth (Meszaros et al. 2014). By 12 weeks after birth, peak expression levels of Bmal1, Per2 and Cry1 were observed during the dark period while peak expression of Rev-erbα occurred during the day. Kidney-specific clock-controlled genes Scnn1a (αENaC), Sgk1 (serum and glucocorticoid-inducible kinase 1), Slc9a3 (NHE3) and Avpr2 (arginine vasopressin receptor 2) showed some rhythmic expression at E20 and these rhythms became more robust one week after delivery in addition to shifting the peak of expression for all four genes by about 12 hours. One week after birth, the peak expression levels of these genes occurred during the day but this again shifted three weeks later and peak expression times shifted closer to the night time, the rats’ active period. Pups were then separated from their mothers between ZT3-7, the time of their highest feeding behavior, for one week after birth. This lead to a 12 hour shift in Bmal1 expression and caused most other genes that were studied to lose their rhythm of expression, possibly due to conflicting light and feeding cues.
Regulation of the kidney clock
After food deprivation for one day and in total darkness, male Wistar rats were allowed access to food for 30 minutes. This feeding stimulus lead to a four hour shift in peak expression of a classic circadian gene, Dbp (D-site albumin binding protein), in the heart but had no effect on expression in the kidney (Wu et al. 2012)). The same study also showed the 30 minute feeding stimulus decreased levels of expression of Bmal1, Cry1, Dbp, Per1 and Per2 in the heart while only Per1 mRNA was significantly decreased in the kidney.
Feeding time also seems to be able to synchronize some peripheral clocks in the absence of a central clock. Bmal1 forebrain KO mice crossed with PER2::LUC mice had no circadian rhythm of PER2::LUC in the kidney in constant darkness but time restricted feeding restored this rhythm in the kidney as well as in the liver (Izumo et al. 2014). This is further supported by the finding that feeding mice only during the day (when they would normally be inactive) shifted the peak of DBP protein levels by 12 hours in the kidney, liver and heart (Damiola et al. 2000). Reversed feeding shifted PER1 and PER2 levels in the liver by 12 hours while there was no effect on either of these proteins in the SCN.
In addition to the time of feeding, specific composition of diets may affect entrainment as well. The type of diet can alter gene expression in order to adapt to changing requirements (Ferraris 2001). Specifically, a high salt (HS) diet causes increased Dbp mRNA expression while decreasing Bmal1 expression in the liver and kidney (Oike et al. 2010). HS diet also advanced the time of peak expression of enzymes involved in glycogen synthesis (Gys2), gluconeogenesis (G6pc) and the synthesis of fatty acids, cholesterol and bile acid (Fasn, Hmgcr and Cyp7a1, respectively). Even though HS diet affected many genes important to metabolism, activity, feeding and drinking behavior were not affected. Timing of food intake and food content both appear to be important cues with respect to the entrainment of peripheral clocks.
Although it is clear that food is a dominant Zeitgeber for peripheral clock entrainment, the dominant cue for the central clock is light. The effects of reversing the LD cycle, restricting time of feeding or changing both the light cycle and feeding times were investigated specifically in the kidney (Wu et al. 2010). LD reversal did not alter the circadian pattern of expression of Bmal1, Cry1, Clock or Per2 mRNA but did delay the peak expression of Per1 by 4 hours. In addition, the amplitude of expression of Per1, Cry1, Clock and Bmal1 were altered only with the reversal of the LD cycle. Shifting feeding time by 12 hours caused 8-12 hour shifts in the peak expression of Clock, Cry1 and Bmal1 after 7 days while causing 4 hour shifts in Per1 and Per2. After 7 days of LD and feeding time reversal, a total inversion of expression of all 5 genes occurred. Thus, separately, light and food cues partially entrain the circadian clock while both synchronize the clock throughout the whole body. Together these results suggest that food cues are a critical synchronizing signal for the kidney clock.
Transgenic PER2::LUC mice were further used to study the effects of temperature on time-dependent entrainment (Ohnishi et al. 2014). These mice were placed in water baths at 35°C, 37°C and 41°C. The treatment at 41°C led to phase advancement by about four hours in the kidney and liver after 1-2 days but peak PER2::LUC expression started to return to normal after repeated treatments for more than two days. After a 2 hour treatments at 41°C, PER2::LUC expression levels were also increased in the kidney and liver. Thus, temperature appears to be another important cue for peripheral clock entrainment.
The circadian clock is classically considered to be temperature compensated, meaning that the free-running period, associated with translation and degradation of clock proteins, does not change much with changes in temperature. This is an intriguing piece of the circadian clock puzzle as the rate of enzymatic reactions should increase with temperature (Segel 1975). However, other experiments in PER2::LUC mice suggest that the SCN is not affected by temperature while peripheral clocks are more susceptible to temperature-induced changes in PER2::LUC expression and this may be mediated by the heat shock pathway (Buhr et al. 2010).
In a more recent study, this same mouse model was used to investigate the effect of sleep deprivation on PER2. Sleep deprivation for 6 hours led to increased PER2::LUC levels in the brain, liver and kidney (Curie et al. 2015). Thus, even relatively short disruptions in the sleep pattern can lead to disrupted circadian rhythms of PER2::LUC protein levels in peripheral tissues including the kidney.
Potassium
Potassium is another urinary electrolyte that has been shown to be regulated by the circadian clock and potassium excretion oscillates with sodium in multiple species (reviewed in (Gumz and Rabinowitz 2013) (Gumz et al. 2015)). In the first study of its kind, Firsov and colleagues performed microarray analysis to characterize circadian gene expression in the kidney, revealing that the expression of many renal genes exhibited time-dependent changes in expression (Zuber et al. 2009) (Nikolaeva et al. 2012), including many genes involved in potassium handling (reviewed in (Gumz and Rabinowitz 2013)). Also like sodium, potassium plays an important role in blood pressure. In addition to disrupted patterns of potassium excretion in diseases such as CKD, potassium supplementation is beneficial in the treatment of hypertension (Zicha et al. 2011) (Kanbay et al. 2013). It has been shown that potassium supplementation leads to increased sodium excretion and therefore decreased blood pressure. In addition to decreasing blood pressure, potassium may also be beneficial in restoring a night time dip in blood pressure, thus reducing the risk of cardiovascular events.
Crambert and colleagues investigated the mechanism of regulation of potassium reabsorption using WT mice, Clock KO mice or mice null for the α subunit of the non-gastric H,K-ATPase (HKα2, encoded by Atp12a) (Salhi et al. 2012). In WT mice, HKα2 was present in significantly higher amounts in the luminal membrane at the end of the rest period while there were no sufficient differences in Clock KO mice. HKα2 deficient mice excreted more potassium compared to WTs during the rest period but they had no differences in sodium excretion. On a low potassium diet, HKα2 mRNA expression peaked about 9 hours later than controls and amplitude of expression increased by 45%. This diet also affected expression of Nrf2, a transcription factor which participates in HKα2 mRNA expression. In controls, expression preceded peak HKα2 mRNA expression by about 4 hours. On a low potassium diet, peak Nrf2 expression was delayed by about 10 hours, again peaking before HKα2 mRNA.
ERRβ is a nuclear receptor specific to the TAL which regulates NKCC2 (Krid et al. 2012). ERRβ mRNA expression is circadian in male CD1 mice with expression peaking at ZT4. Furthermore, this rhythm is lost in mice without Clock. Blockade of ERRβ increased the Na/K urinary excretion ratio and decreased NKCC2 mRNA expression and activity, suggesting a positive regulation of NKCC2. NKCC2 expression is also circadian in mouse kidney, with a peak early in the light period (CircaDB (Pizarro et al. 2013) ), suggesting that it is under transcriptional regulation by the molecular clock. In addition to complex regulation of sodium balance in the kidney, the circadian clock is also integral to maintenance of potassium levels.
Clinical Implications
Many widely used treatments for hypertension such as ACE inhibitors and Ang II receptor blockers (ARBs) aim to inhibit the RAAS. Due to their demonstrated regulation of renal sodium transporters and blood pressure, clock genes may represent novel targets for hypertension treatment. Another potential hypertension treatment target is the elevated 3β-HSD observed in the Cry1/2 KO mice that exhibit salt-sensitive hypertension.
In addition to targeting clock genes to treat disease, another strategy is to work with the clock. The aim of chronotherapy is to administer medicines at certain times of the day to maximize efficacy and reduce side effects. Many hypertension medications are usually taken in the morning, however convincing evidence concerning the effects of chronotherapy shows the benefit of taking at least one hypertension medication at night (for an excellent review, see (Hermida et al. 2013)). The MAPEC study followed over 2000 hypertensive patients over the course of a median of 5.6 years (Hermida et al. 2010). The study divided the volunteers into two groups, one taking all blood pressure medications upon waking and the other group taking at least one medication at night. The night time dosing group showed significant decreases in nocturnal blood pressure and an increase in dipping prevalence of almost 30% compared to the morning dosing group. At the follow up of the study, the night time dosing group had a lower risk of cardiovascular events. They also had a significantly lowered morbidity and mortality due to cardiovascular disease.
Similar to timing the treatment of hypertension, timing of dialysis treatment was also shown to be important with respect to the overall well-being of patients. People undergoing dialysis have been observed to lose a normal night time peak of melatonin production (Viljoen et al. 1992). This hormone is important to the maintenance of the circadian cycle of sleep (Kim et al. 2015). Normally, melatonin shows a circadian rhythm of expression with high production at night that is suppressed by light. This helps to keep the body in sync with day and night. The loss of this rhythm would imply a dysregulation of the sleep cycle. It has been shown that patients undergoing dialysis during the day experience sleep disturbances and daytime tiredness (Parker 2003), while night time dialysis helps to reduce these effects. The night time dialysis patients also have a restored melatonin peak at night (Koch et al. 2010).
It has also been shown that lack of sleep can cause disturbances in kidney function and blood pressure patterns. Compared to people who slept 8-10 hours at night, those who were sleep deprived (no sleep for the night of the experiment) had a greater than 50% increase in nocturnal urine output (Kamperis et al. 2010). While potassium and sodium excretion were increased in both genders in the sleep deprived group, the effect was greater in men. The sleep deprived group exhibited an attenuation of the normal dipping that was observed in the control group. These observations indicate that disruption of circadian rhythms via sleep deprivation can affect kidney function which has important implications for maintaining overall health.
The circadian clock appears to be an important player in the regulation of the cell cycle and disruption of the molecular clock has been associated with cancer (Masri et al. 2015). Renal cell carcinoma is known to be associated with hypoxic signaling pathways and these pathways have recently been linked with the circadian clock (Mazzoccoli et al. 2014). In an important human study linking dysregulation of circadian clock gene expression to renal cell carcinoma, Mazzoccoli et al. demonstrated that several core clock genes were differentially expressed in tumor samples compared to matched normal tissue (Mazzoccoli et al. 2012). These findings likely have important implications for the treatment of kidney cancer. Further studies exploring the role of the molecular clock in mechanisms underlying renal cell carcinoma are needed.
Future Directions
While it is clear that the circadian clock plays a pivotal role in timed, coordinate regulation of many aspects renal function in order to maintain blood pressure and fluid and ion homeostasis in the body, there is still work to be done to identify the exact mechanism of the kidney clock. In order to be defined as truly circadian, rhythms must persist in constant conditions, such as total darkness. Many of the experiments detailed in this review were performed using animals housed in a 12:12 LD cycle, illustrating daily rhythms. While these experiments are still very informative about the workings of the renal clock, total darkness is a requirement to say these observations are indeed circadian, though the participation of circadian proteins in these processes is not in doubt. However, most daily rhythms are found to be circadian as well (Vitaterna et al. 2001). Furthermore, many experiments utilized global KO animals and are thus unable to provide concrete information about renal-specific mechanisms although they certainly support the role of the renal clock. With the advent of CRISPR-Cas9 technology, cell type- and tissue-specific KOs are possible and will allow us to ascertain the contribution of only certain cells and tissues to a given phenotype. Even though the kidney is critical to blood pressure control, the heart, central nervous system and vasculature also make significant, coordinated contributions to this control as well.
In addition to elucidation of renal clock contributions to disease phenotypes through creation of tissue- and cell-specific KO animal models, there is still work to be done with respect to the clinical advantages of chronotherapy. Even though simply changing the time of dosing with antihypertensive medications or dialysis treatments can be greatly beneficial, this is not recommended in either JNC7 (Chobanian et al. 2003) or JNC8 (James et al. 2014), guidelines provided for the treatment of hypertension.
Conclusion
The circadian clock is important in the maintenance of physiological homeostasis, and the kidney plays a critical role in this process. This is achieved in part through the coordinated, timed regulation of channels and transporters responsible for sodium reabsorption in the kidney. Since the clock plays such an important role in blood pressure homeostasis, disruption of the clock leads to dramatic alterations in blood pressure. Even disruption of the clock in a limited number of specific cells in the kidney is enough to cause a measurable blood pressure phenotype. While it is clear that the kidney is a major regulator of blood pressure, the exact mechanisms by which the clock contributes to this regulation remain to be clearly defined. Nonetheless, knowledge of the clock and coordinate regulation of the central and peripheral clocks may allow for better treatment of hypertension. Chronotherapy and nocturnal dialysis have shown that simply changing the timing of administration of existing treatments for diseases improves effectiveness. Future studies to further delineate the renal cell-type specific role of circadian proteins in the maintenance of electrolyte homeostasis and blood pressure regulation are needed to improve our understanding of how the circadian clock contributes to the regulation of renal function.
References
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16(2):100–4. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. Journal of biological rhythms. 2008;23(1):26–36. doi: 10.1177/0748730407311254. doi:10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, et al. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322(2):730–8. doi: 10.1124/jpet.107.122846. doi:jpet.107.122846 [pii]10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli. Journal of bacteriology. 1997;179(12):4075–9. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny O, Vinciguerra M, Gumz ML, Mazzoccoli G. Molecular bases of circadian rhythmicity in renal physiology and pathology. Nephrol Dial Transplant. 2013;28(10):2421–31. doi: 10.1093/ndt/gft319. doi:10.1093/ndt/gft319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295(4):F1063–70. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–85. doi: 10.1126/science.1195262. doi:10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. Journal of pineal research. 2004;36(3):177–85. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Challet E, Dumont S, Mehdi MK, Allemann C, Bousser T, Gourmelen S, Sage-Ciocca D, Hicks D, Pevet P, Claustrat B. Aging-like circadian disturbances in folate-deficient mice. Neurobiol Aging. 2013;34(6):1589–98. doi: 10.1016/j.neurobiolaging.2012.11.021. doi:10.1016/j.neurobiolaging.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. doi:10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Roson MI, Rodriguez Fermepin M, Fernandez BE. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World journal of nephrology. 2015;4(2):196–212. doi: 10.5527/wjn.v4.i2.196. doi:10.5527/wjn.v4.i2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CR, Whelton PK, Moore MA, Caputo RA, Bledsoe T, Walker WG. Dissociation of the diurnal variation of aldosterone and cortisol in anephric subjects. Kidney Int. 1979;15(6):669–75. doi: 10.1038/ki.1979.87. [DOI] [PubMed] [Google Scholar]
- Curie T, Maret S, Emmenegger Y, Franken P. In Vivo Imaging of the Central and Peripheral Effects of Sleep Deprivation and Suprachiasmatic Nuclei Lesion on PERIOD-2 Protein in Mice. Sleep. 2015 doi: 10.5665/sleep.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104(9):3450–5. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonneville C, Staub O. Participation of the ubiquitin-conjugating enzyme UBE2E3 in Nedd4-2-dependent regulation of the epithelial Na+ channel. Mol Cell Biol. 2004;24(6):2397–409. doi: 10.1128/MCB.24.6.2397-2409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, Kimmitt RA, Brown KE, Kennedy ED, Goddard J, Webb DJ. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension. 2014;64(2):296–304. doi: 10.1161/HYPERTENSIONAHA.114.03533. doi:10.1161/HYPERTENSIONAHA.114.03533. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. doi:10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- Dolatshad H, Cary AJ, Davis FC. Differential expression of the circadian clock in maternal and embryonic tissues of mice. PloS one. 2010;5(3):e9855. doi: 10.1371/journal.pone.0009855. doi:10.1371/journal.pone.0009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummer C, Fiedler F, Konig A, Gerzer R. Urodilatin, a kidney-derived natriuretic factor, is excreted with a circadian rhythm and is stimulated by saline infusion in man. Journal of the American Society of Nephrology : JASN. 1991;1(9):1109–13. doi: 10.1681/ASN.V191109. [DOI] [PubMed] [Google Scholar]
- Ede MC, Faulkner MH, Tredre BE. An intrinsic rhythm of urinary calcium excretion and the specific effect of bedrest on the excretory pattern. Clin Sci. 1972;42(4):433–45. doi: 10.1042/cs0420433. [DOI] [PubMed] [Google Scholar]
- Fang Z, Carlson SH, Peng N, Wyss JM. Circadian rhythm of plasma sodium is disrupted in spontaneously hypertensive rats fed a high-NaCl diet. American journal of physiology Regulatory, integrative and comparative physiology. 2000;278(6):R1490–5. doi: 10.1152/ajpregu.2000.278.6.R1490. [DOI] [PubMed] [Google Scholar]
- Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. The Biochemical journal. 2001;360(Pt 2):265–76. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier I, Ploye F, Cottet-Emard JM, Brun J, Claustrat B. Folate deficiency alters melatonin secretion in rats. The Journal of nutrition. 2002;132(9):2781–4. doi: 10.1093/jn/132.9.2781. [DOI] [PubMed] [Google Scholar]
- Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes & development. 2004;18(12):1397–412. doi: 10.1101/gad.301404. doi:10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4(1):25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gallego MS, Ling BN. Regulation of amiloride-sensitive Na+ channels by endothelin-1 in distal nephron cells. The American journal of physiology. 1996;271(2 Pt 2):F451–60. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- Gava AL, Freitas FP, Balarini CM, Vasquez EC, Meyrelles SS. Effects of 5/6 nephrectomy on renal function and blood pressure in mice. International journal of physiology, pathophysiology and pharmacology. 2012;4(3):167–73. [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. doi:10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Grisk O, Kloting I, Exner J, Spiess S, Schmidt R, Junghans D, Lorenz G, Rettig R. Long-term arterial pressure in spontaneously hypertensive rats is set by the kidney. J Hypertens. 2002;20(1):131–8. doi: 10.1097/00004872-200201000-00019. [DOI] [PubMed] [Google Scholar]
- Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285(4):F664–73. doi: 10.1152/ajprenal.00353.2002. doi:10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- Gumz ML, Rabinowitz L. Role of circadian rhythms in potassium homeostasis. Semin Nephrol. 2013;33(3):229–36. doi: 10.1016/j.semnephrol.2013.04.003. doi:10.1016/j.semnephrol.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumz ML, Rabinowitz L, Wingo CS. An Integrated View of Potassium Homeostasis. N Engl J Med. 2015;373(1):60–72. doi: 10.1056/NEJMra1313341. doi:10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119(8):2423–34. doi: 10.1172/JCI36908. doi:10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real-time monitoring system. The Journal of endocrinology. 2007;193(3):413–20. doi: 10.1677/JOE-07-0044. doi:10.1677/JOE-07-0044. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiology international. 2010;27(8):1629–51. doi: 10.3109/07420528.2010.510230. doi:10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Smolensky MH, Mojon A, Fernandez JR, Crespo JJ, Moya A, Rios MT, Portaluppi F. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol. 2013;9(6):358–68. doi: 10.1038/nrneph.2013.79. doi:10.1038/nrneph.2013.79. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus M. Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Archives of toxicology. 1976;36(3-4):305–16. doi: 10.1007/BF00340536. [DOI] [PubMed] [Google Scholar]
- Hill S, Austin S, Eydmann T, Jones T, Dixon R. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(5):2143–8. doi: 10.1073/pnas.93.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419(6909):841–4. doi: 10.1038/nature01123. doi:10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- Huang XM, Chen WL, Yuan JP, Yang YH, Mei QH, Huang LX. Altered diurnal variation and localization of clock proteins in the remnant kidney of 5/6 nephrectomy rats. Nephrology (Carlton) 2013;18(8):555–62. doi: 10.1111/nep.12111. doi:10.1111/nep.12111. [DOI] [PubMed] [Google Scholar]
- Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife. 2014:3. doi: 10.7554/eLife.04617. doi:10.7554/eLife.04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. doi:10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Jensen LW, Pedersen EB. Nocturnal blood pressure and relation to vasoactive hormones and renal function in hypertension and chronic renal failure. Blood pressure. 1997;6(6):332–42. doi: 10.3109/08037059709062092. [DOI] [PubMed] [Google Scholar]
- Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. American journal of physiology Renal physiology. 2010;299(2):F404–11. doi: 10.1152/ajprenal.00126.2010. doi:10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- Kanbay M, Bayram Y, Solak Y, Sanders PW. Dietary potassium: a key mediator of the cardiovascular response to dietary sodium chloride. Journal of the American Society of Hypertension : JASH. 2013;7(5):395–400. doi: 10.1016/j.jash.2013.04.009. doi:10.1016/j.jash.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004;26(2):177–89. doi: 10.1081/ceh-120028556. [DOI] [PubMed] [Google Scholar]
- Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and metabolism. International journal of endocrinology. 2015;2015:591729. doi: 10.1155/2015/591729. doi:10.1155/2015/591729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T. Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications. Sci Signal. 2012;5(244):pt4. doi: 10.1126/scisignal.2003333. doi:10.1126/scisignal.2003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–87. doi: 10.1124/pr.59.3.3. doi:10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Koch BC, Nagtegaal JE, Hagen EC, Wee PM, Kerkhof GA. Different melatonin rhythms and sleep-wake rhythms in patients on peritoneal dialysis, daytime hemodialysis and nocturnal hemodialysis. Sleep Med. 2010;11(3):242–6. doi: 10.1016/j.sleep.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–54. doi: 10.1126/science.1226339. doi:10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool MJ, Wijnen JA, Derkx FH, Struijker Boudier HA, Van Bortel LM. Diurnal variation in prorenin in relation to other humoral factors and hemodynamics. American journal of hypertension. 1994;7(8):723–30. doi: 10.1093/ajh/7.8.723. [DOI] [PubMed] [Google Scholar]
- Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989;77(1):105–11. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- Krid H, Dorison A, Salhi A, Cheval L, Crambert G. Expression profile of nuclear receptors along male mouse nephron segments reveals a link between ERRbeta and thick ascending limb function. PLoS One. 2012;7(3):e34223. doi: 10.1371/journal.pone.0034223. doi:10.1371/journal.pone.0034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Campbell CR, Song SH, Day ML, Kumar S, Cook DI, Dinudom A. The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J Biol Chem. 2009;284(19):12663–9. doi: 10.1074/jbc.M809737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Hirota T, Peters EC, Garcia M, Gonzalez R, Cho CY, Wu X, Schultz PG, Kay SA. A small molecule modulates circadian rhythms through phosphorylation of the period protein. Angewandte Chemie. 2011;50(45):10608–11. doi: 10.1002/anie.201103915. doi:10.1002/anie.201103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliavski A, Dumbell R, Ott V, Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms. 2015;30(1):20–34. doi: 10.1177/0748730414553971. doi:10.1177/0748730414553971. [DOI] [PubMed] [Google Scholar]
- Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel) J Biol Chem. 2005;280(45):37717–24. doi: 10.1074/jbc.M506397200. doi:M506397200 [pii]10.1074/jbc.M506397200. [DOI] [PubMed] [Google Scholar]
- Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ET(A) and ET(B) receptors in the mouse cortical collecting duct. American journal of physiology Renal physiology. 2013;305(4):F568–73. doi: 10.1152/ajprenal.00613.2012. doi:10.1152/ajprenal.00613.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Kinouchi K, Sassone-Corsi P. Circadian clocks, epigenetics, and cancer. Current opinion in oncology. 2015;27(1):50–6. doi: 10.1097/CCO.0000000000000153. doi:10.1097/CCO.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G, De Cata A, Piepoli A, Vinciguerra M. The circadian clock and the hypoxic response pathway in kidney cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(1):1–7. doi: 10.1007/s13277-013-1076-5. doi:10.1007/s13277-013-1076-5. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Piepoli A, Carella M, Panza A, Pazienza V, Benegiamo G, Palumbo O, Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother. 2012;66(3):175–9. doi: 10.1016/j.biopha.2011.11.007. doi:10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Meszaros K, Pruess L, Szabo AJ, Gondan M, Ritz E, Schaefer F. Development of the circadian clockwork in the kidney. Kidney Int. 2014;86(5):915–22. doi: 10.1038/ki.2014.199. doi:10.1038/ki.2014.199. [DOI] [PubMed] [Google Scholar]
- Mills JN, Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. The Journal of physiology. 1952;117(1):22–37. [PMC free article] [PubMed] [Google Scholar]
- Moon JY. Recent Update of Renin-angiotensin-aldosterone System in the Pathogenesis of Hypertension. Electrolyte Blood Press. 2013;11(2):41–5. doi: 10.5049/EBP.2013.11.2.41. doi:10.5049/EBP.2013.11.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol. 1986;250(5 Pt 2):R737–52. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- Moore Ede MC, Brennan MF, Ball MR. Circadian variation of intercompartmental potassium fluxes in man. J Appl Physiol. 1975;38(1):163–70. doi: 10.1152/jappl.1975.38.1.163. [DOI] [PubMed] [Google Scholar]
- Morawska-Barszczewska J, Guzek JW, Kaczorowska-Skora J. Cholecystokinin octapeptide and the daily rhythm of vasopressin and oxytocin release. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 1996;104(2):164–71. doi: 10.1055/s-0029-1211439. doi:10.1055/s-0029-1211439. [DOI] [PubMed] [Google Scholar]
- Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23(6):1019–26. doi: 10.1681/ASN.2011080842. doi:10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishide SY, Hashimoto K, Nishio T, Honma K, Honma S. Organ-specific development characterizes circadian clock gene Per2 expression in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(1):R67–74. doi: 10.1152/ajpregu.00063.2013. doi:10.1152/ajpregu.00063.2013. [DOI] [PubMed] [Google Scholar]
- Noshiro M, Furukawa M, Honma S, Kawamoto T, Hamada T, Honma K, Kato Y. Tissue-specific disruption of rhythmic expression of Dec1 and Dec2 in clock mutant mice. J Biol Rhythms. 2005;20(5):404–18. doi: 10.1177/0748730405280195. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. Journal of hypertension. 2002;20(11):2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- Ohnishi N, Tahara Y, Kuriki D, Haraguchi A, Shibata S. Warm water bath stimulates phase-shifts of the peripheral circadian clocks in PER2::LUCIFERASE mouse. PLoS One. 2014;9(6):e100272. doi: 10.1371/journal.pone.0100272. doi:10.1371/journal.pone.0100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Honma S, Abe H, Honma K. Effects of nursing mothers on rPer1 and rPer2 circadian expressions in the neonatal rat suprachiasmatic nuclei vary with developmental stage. The European journal of neuroscience. 2002;15(12):1953–60. doi: 10.1046/j.1460-9568.2002.02016.x. [DOI] [PubMed] [Google Scholar]
- Oike H, Nagai K, Fukushima T, Ishida N, Kobori M. High-salt diet advances molecular circadian rhythms in mouse peripheral tissues. Biochemical and biophysical research communications. 2010;402(1):7–13. doi: 10.1016/j.bbrc.2010.09.072. doi:10.1016/j.bbrc.2010.09.072. [DOI] [PubMed] [Google Scholar]
- Oster H, Baeriswyl S, Van Der Horst GT, Albrecht U. Loss of circadian rhythmicity in aging mPer1−/−mCry2−/− mutant mice. Genes & development. 2003;17(11):1366–79. doi: 10.1101/gad.256103. doi:10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur S, Sancar A. Purification and properties of human blue-light photoreceptor cryptochrome 2. Biochemistry. 2003;42(10):2926–32. doi: 10.1021/bi026963n. doi:10.1021/bi026963n. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Schnermann J. Integrated Control of Na Transport along the Nephron. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(4):676–87. doi: 10.2215/CJN.12391213. doi:10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KP. Sleep disturbances in dialysis patients. Sleep medicine reviews. 2003;7(2):131–43. doi: 10.1053/smrv.2001.0240. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends in cell biology. 2014;24(2):90–9. doi: 10.1016/j.tcb.2013.07.002. doi:10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41:D1009–13. doi: 10.1093/nar/gks1161. Database issue. doi:10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M, Forpomes O, Espagnet S, Cambar J. Relationship between circadian changes in renal hemodynamics and circadian changes in urinary glycosaminoglycan excretion in normal rats. Chronobiology international. 1996;13(5):349–58. doi: 10.3109/07420529609012659. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Bagni B, degli Uberti E, Montanari L, Cavallini R, Trasforini G, Margutti A, Ferlini M, Zanella M, Parti M. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. Journal of hypertension. 1990;8(1):85–95. doi: 10.1097/00004872-199001000-00013. [DOI] [PubMed] [Google Scholar]
- Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. Pflugers Arch. 2013;465(1):121–32. doi: 10.1007/s00424-012-1151-6. doi:10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Richards J, All S, Skopis G, Cheng KY, Compton B, Srialluri N, Stow L, Jeffers LA, Gumz ML. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. American journal of physiology Regulatory, integrative and comparative physiology. 2013a;305(7):R735–47. doi: 10.1152/ajpregu.00195.2013. doi:10.1152/ajpregu.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Cheng KY, All S, Skopis G, Jeffers L, Jeanette Lynch I, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol. 2013b;305(12):F1697–704. doi: 10.1152/ajprenal.00472.2013. doi:10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol. 2013c;305(12):F1697–704. doi: 10.1152/ajprenal.00472.2013. doi:10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1delta/epsilon. Am J Physiol Renal Physiol. 2012;303(7):F918–27. doi: 10.1152/ajprenal.00678.2011. doi:10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26(9):3602–13. doi: 10.1096/fj.12-203554. doi:10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Jeffers LA, All SC, Cheng KY, Gumz ML. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of alphaENaC in renal cortical collecting duct cells. Front Physiol. 2013d;4:253. doi: 10.3389/fphys.2013.00253. doi:10.3389/fphys.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem. 2014a;289(17):11791–806. doi: 10.1074/jbc.M113.531095. doi:10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Welch AK, Barilovits SJ, All S, Cheng KY, Wingo CS, Cain BD, Gumz ML. Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sci. 2014b;118(2):255–62. doi: 10.1016/j.lfs.2014.03.028. doi:10.1016/j.lfs.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig S, Matthiesen TB, Pedersen EB, Djurhuus JC. Circadian variation of angiotensin II and aldosterone in nocturnal enuresis: relationship to arterial blood pressure and urine output. The Journal of urology. 2006;176(2):774–80. doi: 10.1016/S0022-5347(06)00594-5. doi:10.1016/S0022-5347(06)00594-5. [DOI] [PubMed] [Google Scholar]
- Rossier BC. Epithelial sodium channel (ENaC) and the control of blood pressure. Current opinion in pharmacology. 2014;15:33–46. doi: 10.1016/j.coph.2013.11.010. doi:10.1016/j.coph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int. 2005;67(4):1410–9. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- Salhi A, Centeno G, Firsov D, Crambert G. Circadian expression of H,K-ATPase type 2 contributes to the stability of plasma K+ levels. FASEB J. 2012 doi: 10.1096/fj.11-199711. doi:fj.11-199711 [pii]10.1096/fj.11-199711. [DOI] [PubMed] [Google Scholar]
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Steady State and Steady-State Enzyme Systems. Wiley; New York: 1975. [Google Scholar]
- Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocrine reviews. 2005;26(4):525–82. doi: 10.1210/er.2002-0050. doi:10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- Stefanovic V, Ivic M, Mitic M. Diurnal urinary excretion of cortisol and aldosterone in kidney graft recipients. Pathol Biol (Paris) 1995;43(9):766–71. [PubMed] [Google Scholar]
- Stepien M, Witte K, Lemmer B. Chronobiologic evaluation of angiotensin-converting enzyme activity in serum and lung tissue from normotensive and spontaneously hypertensive rats. Chronobiology international. 1993;10(5):331–7. doi: 10.3109/07420529309064487. [DOI] [PubMed] [Google Scholar]
- Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22(4):598–604. doi: 10.1681/ASN.2010080803. doi:10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59(6):1151–6. doi: 10.1161/HYPERTENSIONAHA.112.190892. doi:10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lu B, Li RQ, Flavell RA, Taneja R. Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nature immunology. 2001;2(11):1040–7. doi: 10.1038/ni721. doi:10.1038/ni721. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yang Z, Niu Z, Wang W, Peng J, Li Q, Ma MY, Zhao Y. The mortality of MOP3 deficient mice with a systemic functional failure. Journal of biomedical science. 2006;13(6):845–51. doi: 10.1007/s11373-006-9108-4. doi:10.1007/s11373-006-9108-4. [DOI] [PubMed] [Google Scholar]
- Susa K, Sohara E, Isobe K, Chiga M, Rai T, Sasaki S, Uchida S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem Biophys Res Commun. 2012;427(4):743–7. doi: 10.1016/j.bbrc.2012.09.130. doi:10.1016/j.bbrc.2012.09.130. [DOI] [PubMed] [Google Scholar]
- Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol. 2014;25(7):1430–9. doi: 10.1681/ASN.2013060641. doi:10.1681/ASN.2013060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature genetics. 2005;37(2):187–92. doi: 10.1038/ng1504. doi:10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- Vesely DL. Urodilatin: a better natriuretic peptide? Current heart failure reports. 2007;4(3):147–52. doi: 10.1007/s11897-007-0033-2. [DOI] [PubMed] [Google Scholar]
- Vesely DL, Sothern RB, Scheving LE, Bremner FW, Third JL, McCormick JB, Dawson S, Kahn S, Augustine G, Ryan M, et al. Circadian relationships between circulating atrial natriuretic peptides and serum calcium and phosphate in healthy humans. Metabolism: clinical and experimental. 1996;45(8):1021–8. doi: 10.1016/s0026-0495(96)90274-7. [DOI] [PubMed] [Google Scholar]
- Viljoen M, Steyn ME, van Rensburg BW, Reinach SG. Melatonin in chronic renal failure. Nephron. 1992;60(2):138–43. doi: 10.1159/000186729. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25(2):85–93. [PMC free article] [PubMed] [Google Scholar]
- Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(3):R627–34. doi: 10.1152/ajpregu.00404.2009. [DOI] [PubMed] [Google Scholar]
- Walker BR, Campbell JC, Fraser R, Stewart PM, Edwards CR. Mineralocorticoid excess and inhibition of 11 beta-hydroxysteroid dehydrogenase in patients with ectopic ACTH syndrome. Clin Endocrinol (Oxf) 1992;37(6):483–92. doi: 10.1111/j.1365-2265.1992.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Maillard M, Schibler U, Burnier M, Gachon F. Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299(4):R1013–9. doi: 10.1152/ajpregu.00241.2010. doi:ajpregu.00241.2010 [pii]10.1152/ajpregu.00241.2010. [DOI] [PubMed] [Google Scholar]
- Weinert D. Age-dependent changes of the circadian system. Chronobiology international. 2000;17(3):261–83. doi: 10.1081/cbi-100101048. [DOI] [PubMed] [Google Scholar]
- Winters CJ, Sallman AL, Vesely DL. Circadian rhythm of prohormone atrial natriuretic peptides 1-30, 31-67 and 99-126 in man. Chronobiology international. 1988;5(4):403–9. doi: 10.3109/07420528809067785. [DOI] [PubMed] [Google Scholar]
- Wu T, Fu O, Yao L, Sun L, Zhuge F, Fu Z. Differential responses of peripheral circadian clocks to a short-term feeding stimulus. Molecular biology reports. 2012;39(10):9783–9. doi: 10.1007/s11033-012-1844-0. doi:10.1007/s11033-012-1844-0. [DOI] [PubMed] [Google Scholar]
- Wu T, Ni Y, Dong Y, Xu J, Song X, Kato H, Fu Z. Regulation of circadian gene expression in the kidney by light and food cues in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(3):R635–41. doi: 10.1152/ajpregu.00578.2009. [DOI] [PubMed] [Google Scholar]
- Wu T, Ni Y, Zhuge F, Sun L, Xu B, Kato H, Fu Z. Significant dissociation of expression patterns of the basic helix-loop-helix transcription factors Dec1 and Dec2 in rat kidney. The Journal of experimental biology. 2011;214(Pt 8):1257–63. doi: 10.1242/jeb.052100. doi:10.1242/jeb.052100. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, Soulsby S, Papadimas E, Pezuk P, Doyle SE, Tei H, et al. Ontogeny of circadian organization in the rat. J Biol Rhythms. 2009;24(1):55–63. doi: 10.1177/0748730408328438. doi:10.1177/0748730408328438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–24. doi: 10.1073/pnas.1408886111. doi:10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–73. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- Zicha J, Dobesova Z, Behuliak M, Kunes J, Vaneckova I. Preventive dietary potassium supplementation in young salt-sensitive Dahl rats attenuates development of salt hypertension by decreasing sympathetic vasoconstriction. Acta physiologica. 2011;202(1):29–38. doi: 10.1111/j.1748-1716.2010.02248.x. doi:10.1111/j.1748-1716.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106(38):16523–8. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]