Abstract
Hydroxyurea (HU) is a crucial therapy for children with sickle cell anemia, but its off-label use is a barrier to widespread acceptance. We found HU exposure is not significantly altered by liquid vs capsule formulation, and weight-based dosing schemes provide consistent exposure. HU is recommended for all children starting as young as 9 months of age with sickle cell anemia (SCA; HbSS and HbSβspan0thalassemia); however; a paucity of pediatric data exists regarding the pharmacokinetics (PK) or the exposure-response relationship of HU. This trial aimed to characterize the PK of HU in children and to evaluate and compare the bioavailability of a liquid vs capsule formulation. This multicenter; prospective; open-label trial enrolled 39 children with SCA who provided 682 plasma samples for PK analysis following administration of HU. Noncompartmental and population PK models are described. We report that liquid and capsule formulations of HU are bioequivalent; weight-based dosing schemes provide consistent drug exposure; and age-based dosing schemes are unnecessary. These data support the use of liquid HU in children unable to swallow capsules and in those whose weight precludes the use of fixed capsule formulations. Taken with existing safety and efficacy literature; these findings should encourage the use of HU across the spectrum of age and weight in children with SCA; and they should facilitate the expanded use of HU as recommended in the National Heart; Lung; and Blood Institute guidelines for individuals with SCA.
Keywords: bioequivalent, hydroxyurea. children, sickle cell anemia
Sickle cell anemia (SCA; HbSS and HbSβ0thalassemia) is a chronic, debilitating, and costly disease affecting an estimated 100,000 individuals in the United States.1 Hydroxyurea (HU), a ribonucleotide reductase inhibitor, is a potent fetal hemoglobin inducer and antisickling agent. In 1998, HU received approval by the US Food and Drug Administration (FDA) for adults with SCA, largely based on data generated from the Multicenter Study of Hydroxyurea in Patients with Sickle Cell Anemia (MSH) trial (NCT00000586). The MSH trial showed that HU was safe and significantly decreases the incidence of pain, acute chest syndrome, hospitalization, and transfusion in adults with severe SCA.2
An estimated 36,000 children (<19 years old) suffer from SCA in the United States.1 Since its approval for adult usage, HU has been prescribed off-label for children with SCA to ameliorate their disease, and the laboratory and clinical benefits in children parallel those seen in adults with reductions in pain, acute chest syndrome, hospitalizations, and transfusion requirements.3–9 In September 2014, an expert panel selected by the National Heart, Lung, and Blood Institute (NHLBI) released evidence-based management guidelines for sickle cell disease. Included in these guidelines was a “strong recommendation” for children ≥9 to ≤42 months of age and a “moderate recommendation” for children >42 months of age to be offered HU therapy to reduce complications related to SCA.10
Despite supporting data and recommendations, pediatric use continues to be off-label. FDA approval requires additional pharmacokinetic (PK) information to include a pediatric indication on the label for HU and approve it for pediatric use. Although efficacy and toxicity profiles of HU in children with SCA have been favorable,8,9,11,12 limited data exist regarding the PK or exposure-response relationship. The FDA offered a written request under the Best Pharmaceuticals for Children Act (BPCA) to specifically address this knowledge gap and highlighted the need for PK data to compare liquid to capsule formulations.
In response to this written request, the “Pharmacokinetics and Bioavailability of a Liquid Formulation of Hydroxyurea in Pediatric Patients with Sickle Cell Anemia” trial (NCT01506544) was designed to characterize the disposition of a liquid HU formulation in a cohort of toddlers (≥2 to ≤5 years). This study had two hypotheses: (1) the PK in toddlers administered a liquid formulation would not differ significantly from that observed in older children; and (2) the PK profile of the liquid formulation would not differ significantly from the PK profile of a proprietary capsule formulation.
Methods
Study Population
Following approval from each local center’s institutional review board, children ≥2 to ≤17 years of age were recruited from 7 medical centers in the United States. Eligible participants had either HbSS or HbSβ0 thalassemia. The inclusion criteria included being in a “well” state (ie, no acute SCA manifestations or other acute illness), weight of ≥10 kg, body mass index (BMI) ≥5th and ≤95th percentiles, and normal hepatic, renal, and gastrointestinal function. Children were ineligible if they had a malignancy, had received a recent blood transfusion, or had cytopenia noted on screening laboratory assessment. Additionally, children were excluded for concomitant medication usage that could potentially affect the analysis (Tables S1 and S2). Of note, 90% (n = 35/39) of children were actively being treated with HU.
Study Design
This was a prospective, open-label, trial composed of 2 arms that assessed the PK parameters of HU administered under direct supervision. Participants were allowed only clear liquids from 6 hours to 1 hour prior to HU administration, and they abstained from food or drink 1 hour before and 1 hour following drug administration. A regular age-appropriate diet was resumed 2 hours after dosing. In arm 1, toddlers (≥2 to ≤5 years) (n = 17) received a single dose of a standardized liquid formulation of HU (100 mg/mL). In arm 2, children (>5 to ≤17 years) (n = 22) received each standardized formulation (liquid and capsule) on separate occasions in a randomized, crossover fashion. The second dose was administered between 24 hours and 10 days following the initial dose. Following all HU dosing, blood PK samples were collected predose and then at 10, 15, 30, 45, and 60 minutes, and 1.5, 2, 4, 6, and 8 hours postdose in both arms. To ensure balanced enrollment, arm 1 was subdivided into ages 2–3 years and 4–5 years, and arm 2 was divided into ages 6–11 years and 12–17 years.
Investigational Products
Hydroxyurea USP was purchased from a commercial pharmaceutical company and reconstituted with a commercially available cherry syrup diluent to a final concentration of 100 mg/mL at each site’s investigational pharmacy. Droxia® capsules (200 mg) were purchased and supplied to study sites without modification or disturbance of the commercially obtained bottles. Capsules were administered whole and were not opened. Children received HU at a dose of either 20 mg/kg if they were HU naive or their normal daily dosage (rounded to the nearest 200 mg and no greater than 30 mg/kg) for children currently prescribed HU therapy.
Bioanalytical Methods
Whole blood (1 mL) was collected into heparinized tubes, inverted a minimum of 8 to 10 times, placed immediately on ice, and centrifuged at 750g for 10 minutes at 4°C. Plasma was then stored at −70°C until shipped to Children’s Mercy Hospital, where it was analyzed. PK samples were analyzed using a validated gas chromatography-mass spectrometry (GC-MS) technique that was linear from 0.1 to 100 μg/mL of HU.13 The intra- and interassay coefficients of variation (CV) were consistently <10% across concentrations spanning the range of linearity.14
Bioavailability and a Noncompartmental PK Method
A noncompartmental PK analysis was performed using WinNonlin software (Certera, Princeton, New Jersey). All PK parameters were expressed with descriptive statistics of arithmetic mean, standard deviation (SD), and coefficient of variation (CV%).
The assessment of bioavailability followed FDA guidelines.15 The geometric least-squares (LS) means of the maximum observed plasma concentration (Cmax), the area under the concentration-vs-time curve calculated using the log-linear trapezoidal method from time 0 to the last quantifiable concentration (AUClast), and the area under the concentration-vs-time curve calculated using the log-linear trapezoidal method from time 0 extrapolated to time infinity (AUC∞) were generated using WinNonlin. The ratio of these parameters for liquid: capsule and their 90% confidence limits were also obtained using WinNonlin. The paired t-test used to compare Cmax, AUClast, AUC∞, and time to maximum plasma concentration (Tmax) and the assessment of mean (SD; range) was calculated using Microsoft Excel 2007.
Population Pharmacokinetic Models
A 1-compartment model with absorption described by a series of transit compartments was fitted to the concentration-time profiles of HU using the following equations:
| (1) |
where A is the amount of HU in the central compartment, CL is the systemic clearance, V is the volume of distribution, F is the fraction of the dose absorbed, plasma concentrations (Cp) = A/V, and Input(t) denotes the input rate of HU into the central compartment:
| (2) |
where t is the time since the first dose, Dosek denotes the dose given at kth visit (occasion) at time TODk, and I(x,y) is the individual input function defined as:
| (3) |
where ktr is the first-order transit rate constant equal to (N+1)/MTT; MTT (mean transit time) corresponds to the average time spent by a molecule traveling from the dirst to the central compartment (average absorption time), and N is number of transit compartments. The actual parameters generated were CL/F and V/F due to uncertain bioavailability. Equations 1–3 allow for joint estimation of the number of compartments and MTT. Interindividual variability (IIV) represented by η and interoccasion variability (IOV) represented by κ were modeled assuming the log normal distribution. Model fitting and covariate analysis were performed using NONMEM software (version 7.2.0, Icon Development Solutions, Ellicott City, Maryland) as described in the supplemental materials.
Allometric Scaling
The effect of body size on the volume and clearance parameters was predicated on allometric principles with theoretical exponents as follows:
| (4) |
| (5) |
where Vi and CLi denote the individual values of volume and clearance; θV and θCL are the population (typical) estimates of volume and clearance, BWi,k is the individual body weight on the kth occasion (visit), and 70 is the body weight (kg) of a standard adult. This approach facilitated the comparison of PK parameters with adult data.
Covariance Analysis
Relationships between patient-specidic covariates (such as age, sex, height, alkaline phosphate, alanine transaminase, aspartate transaminase, direct bilirubin, blood urine nitrogen, creatinine, glucose, potassium, sodium, and sickle cell genotype) and the empirical Bayes estimates of η and κ for each PK parameter were sought by plotting individual estimates of η and κ against tested covariates to identify their indluence. Potential relationships were subsequently evaluated using nonlinear mixed-effect modeling with the stepwise forward inclusion (P < .05) and backward elimination (P < .005) method. Further details regarding covariate assessment are provided in the supplemental materials.
Results
In total, 39 participants enrolled. In arm 1, 94% (n = 16/ 17) of young children (n = 6, aged 2–3 years; n = 10, aged 4–5 years) received study drug. All 16 children completed the study and are included in the PK and safety analyses. In arm 2, 25 children were enrolled, and 92% (n = 23; 12 aged 6–11 years, 11 aged 12–17 years) received study drug and are included in the PK and safety analyses. For the bioavailability analysis of participants in arm 2, 96% of children (n = 22) completed both PK visits, with 48% (n = 11) receiving capsule formulation first. One participant voluntarily withdrew from the second PK analysis due to loss of intravenous access. The PK samples obtained with the study drug (liquid formulation) during this child’s first PK visit were analyzed in the PK and safety analysis. Table 1 summarizes demographic, baseline laboratory parameters, and HU dose by study arm and participant age.
Table 1.
Demographic, Baseline Laboratory Parameters, and Hydroxyurea Dosage by Study Arm
| Category; Mean (SD) | Arm 1, n = 16
|
Arm 2, n = 23
|
||
|---|---|---|---|---|
| 2–3 Years
|
4–5 Years
|
6–11 Years
|
12–17 Years
|
|
| n = 6 | n = 10 | n = 12 | n = 11 | |
| Demographics | ||||
| Age, years | 3.1 (0.5) | 4.8 (0.5) | 9.0 (1.4) | 15.1 (2.0) |
| Height, cm | 96.3 (4.8) | 109.5 (5.2) | 132.4 (7.0) | 162.5 (9.8) |
| Weight, kg | 14.8 (1.4) | 18.2 (2.2) | 28.2 (4.9) | 53.3 (13.9) |
| Baseline laboratory parameters | ||||
| WBC, 103/L | 11.9 (3.2) | 10.6 (4.0) | 9.4 (3.7) | 6.9 (2.3) |
| Hb, g/L | 87.7 (15.8) | 86.9 (10.2) | 87.8 (9.7) | 93.6 (19.0) |
| MCV, fL | 90.4 (8.6) | 93.9 (12.5) | 91.9 (8.9) | 95.2 (14.0) |
| Platelets, 103/L | 432 (172) | 402 (167) | 397 (116) | 372 (208) |
| Sodium, mmol/L | 140.0 (1.9) | 138.0 (1.8) | 139.6 (2.3) | 141.8 (3.1) |
| Potassium, mmol/L | 4.5 (0.6) | 4.3 (0.2) | 4.3 (0.3) | 4.2 (0.5) |
| BUN, mmol/L | 2.6 (1.3) | 3.1 (0.9) | 3.2 (1.0) | 2.6 (0.9) |
| Creatinine, μmol/L | 23.8 (11.4) | 28.6 (5.1) | 36.0 (7.8) | 43.3 (10.9) |
| ALP, U/L | 178.7 (30.5) | 153.3 (25.1) | 167.8 (65.0) | 135.4 (56.1) |
| AST, U/L | 47.2 (11.8) | 43.2 (13.4) | 51.1 (17.2) | 49.7 (20.1) |
| BiliT, μmol/L | 33.1 (12.7) | 31.8 (14.4) | 45.0 (20.7) | 34.5 (16.2) |
| Hydroxyurea dose, mg/kg | ||||
| Liquid | 23.6 (3.0) | 24.1 (4.2) | 21.0 (4.5) | 24.4 (4.9) |
| Capsule | N/A | N/A | 21.3 (4.6) | 24.5 (5.1) |
SD indicates standard deviation; WBC, white blood cell; Hb, hemoglobin; MCV, mean corpuscular volume; BUN, blood urea nitrogen; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BiliT, total bilirubin; N/A, not applicable.
Bioavailability
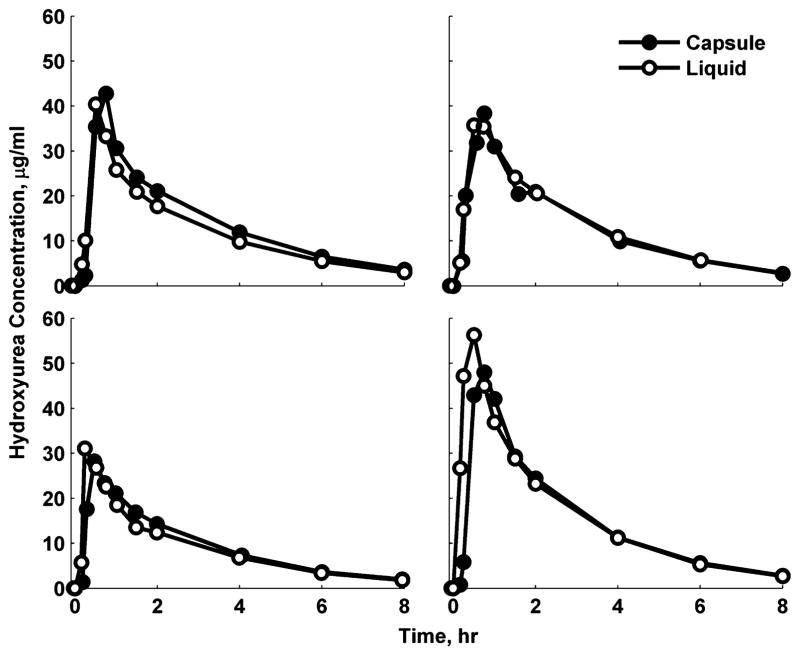
The PK of liquid (n = 22) and capsule (n = 22) formulations were similar (Table 2). Figure 1 shows HU concentration-vs-time profiles in 4 representative children who received both liquid and capsule formulations. Both formulations were rapidly absorbed with a mean (SD) Tmax of 0.86 (0.53) hours and resulted in a Cmax of 33.8 (8.3) μg/mL. The half-life was 2.3 (0.5) hours, and clearance averaged 0.20 (0.03) L/(h · kg). Comparing the capsule and liquid formulations using the geometric least-squares mean ratios of the 3 bioequivalence metrics (Cmax, AUClast, and AUC∞), the formulations were nearly 100% identical and well within the 90% confidence limits (Table 3).
Table 2.
Pharmacokinetic Parameters in Children in Arm 2 for Capsule and Liquid Formulations of Hydroxyurea
| Parameters, Mean (SD) | Both n = 44 |
Capsule n = 22 |
Liquid n = 22 |
|---|---|---|---|
| Cmax (μg/mL) | 33.8 (8.3) | 33.6 (8.2) | 34.0 (8.7) |
| Tmax (hour) | 0.86 (0.53) | 0.97 (0.52) | 0.74 (0.5) |
| AUClast (μg · h/mL) | 101.7 (24.7) | 103.0 (25.4) | 100.3 (24.6) |
| AUC∞ (μg ·h/mL) −1) | 114.1 (29.4) | 116.3 (30.0) | 111.9 (29.4) |
| λz (hour | 0.31 (0.05) | 0.31 (0.05) | 0.31 (0.05) |
| t½(hours) | 2.3 (0.5) | 2.3 (0.5) | 2.3 (0.5) |
| MRT (hours) | 2.8 (0.4) | 2.9 (0.4) | 2.8 (0.4) |
| CL/F/BW (L/[hr · kg]) | 0.20 (0.03) | 0.20 (0.03) | 0.21 (0.04) |
Cmax, maximum observed plasma concentration; Tmax, time to maximum plasma concentration; AUClast, area under the concentration-vs-time curve calculated using the log-linear trapezoidal method from time 0 to the last quantifiable concentration; AUC∞, area under the concentration-vs-time curve calculated using the log-linear trapezoidal method from time 0 extrapolated to time ∞; λz, terminal elimination slope constant; t½, terminal elimination half-life calculated as ln2/λz; MRT, mean residence time; CL/F/ BW, clearance normalized for body weight. Parameters for liquid and capsule formulations compared with a paired 2-tailed t-test, and none were significantly different.
Figure 1.
Representative hydroxyurea concentration (μg/mL) per time (hour) profiles in 4 selected children who were given both liquid (–○–) and capsule (–●–) formulations.
Table 3.
Assessment of Hydroxyurea Bioavailability via FDA Guidelines
| Parameters (Units) | Geometric LS Mean
|
Ratio (%) | 90% Confidence Limits
|
||
|---|---|---|---|---|---|
| Capsule | Liquid | Lower | Upper | ||
| Cmax (μg/mL) | 32.3 | 33.1 | 102.4 | 91.8 | 114.2 |
| AUClast (μg ·h/mL) | 100.3· | 98.6 | 98.3 | 94.5 | 102.3 |
| AUC∞ (μg ·h/mL) | 113.3 | 110.1 | 97.2 | 93.4 | 101.1 |
Abbreviations defined in Table 2; LS indicates least-squares.
Noncompartmental Analysis
Noncompartmental analyses were performed in children (≥2 to ≤5 years) in arm 1 and in the single participant in arm 2 who withdrew prior to completion of the second PK visit as well as in older children (>5 to ≤17 years) in arm 2. In young children, following a mean (SD) dose of 22.7 (3.0) mg/kg of HU, absorption was rapid with a mean (SD) Tmax of 0.57 (0.34) hours. The decline phase after a Cmax of 37.4 (9.3) μg /mL was well captured, and the average half-life was 1.96 (0.18) hours. The mean residence time (MRT) was small, averaging 2.6 (0.3) hours. The apparent clearance was consistent among these subjects, averaging 0.234 (0.028) L/(h ·kg). In older children (arm 2), HU was rapidly absorbed with a Tmax of 0.86 (0.53) hours, mean Cmax was 33.8 (8.3) μg/mL, and the mean half-life was 2.31 (0.47) hours. The MRT was also small, averaging 2.8 (0.4) hours.
Comparison of Pharmacokinetic Studies
Table 4 provides a summary of existing studies assessing the PK properties of HU in patients with SCA. Children from the bioavailability analysis (arm 2) and those from the single-dose (arm 1) analysis are presented separately.
Table 4.
Summary of Published Pharmacokinetic Parameters of Hydroxyurea
| Study | Year | N | Age, Years | Dose, mg/kg | Formulation | Cmax,μg/mL | AUC∞,μg · h/mL | Tmax, hr | t½, hr | CL/F/BW, L/(h · kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | – | 17 | 4.5 (1.7) | 22.7 (3.0) | liquid | 37.4 (9.3) | 104.5 (18.8) | 0.57 (0.34) | 1.96 (0.18) | 0.234 (0.028) |
| Arm 2 | – | 22 | 12.0 (3.6) | 21.7 (6.4) | liquid | 34.0 (8.7) | 111.9 (29.4) | 0.74 (0.52) | 2.3 (0.5) | 0.205 (0.035) |
| Arm 2 | – | 22 | 12.0 (3.6) | 22.6 (4.9) | capsule | 33.6 (8.2) | 116.3 (30.0) | 0.97 (0.52) | 2.31 (0.47) | 0.20 (0.03) |
| Children | ||||||||||
| Rogers et al16 | 2005 | 22 | 1.2 (NR) | 20 | liquid | 19.8 (5.8) | 68.8 (11.5) | NR | 2.36 (0.99) | NR |
| Ware et al17 | 2011 | 87 | 9.6 (4.8) | 20 | liquid | 26.1 (6.8) | 93.0 (23.4) | 0.82 (0.47) | 1.70 (0.53) | 0.24 (0.09) |
| de Montalembert et al18 | 2006 | 11 | 10.2 (5.5) | 21.9 | tablets | 24.5 | 115.8 | 0.75 | 6.3 | 0.127 |
| Adults | ||||||||||
| de Montalembert et al18 | 2006 | 15 | 30.1 | 20.9 | both | 26.5 | 128.4 | 0.86 | 6.3 | 0.154 |
| Yan et al19 | 2005 | 7 | ~40 | 15 | capsule | 28.3 (11.0) | 82.5 (15.5) | 0.5 | 3.14 | 0.194 |
Abbreviations are defined in Table 2; NR indicates not reported. Data presented are mean (standard deviation).
Population Pharmacokinetic Modeling
Individual HU concentration-time profiles are shown in Figure S1. The available data consisted of 682 plasma concentrations with 62 measurements (60 predose) below the level of quantification. Five predose concentrations were above the limit of quantification and were treated as outliers.
A 1-compartment model with first-order absorption was initially used to describe the data. However, delayed absorption in several children required a more flexible model. Therefore, a model with a series of transit compartments describing absorption and a 1-compartment model describing disposition were designed that successfully characterized the trend and variability in the data. Figure S2 demonstrates the goodness-of-fit plots of the final model. The individual and population predictions vs observed concentrations are symmetrically distributed around the line of identity. The visual predictive plots stratified by formulation are presented in Figure S3. Individual predicted concentration-vs-time profiles were very close to the experimental data (Figures S4a–d).
All PK parameters, intersubject, interoccasion, and residual error variances had CVs smaller than 55% (Table S3). The intraindividual variability for the number of transit compartments (N) tended to 0 during the model-building process and was fixed to 0. The intraindividual variability for MTT was not significantly different from 0 as tested by the decrease in the NONMEM objective function and was also fixed to 0. The IOV was tested only for MTT, as the absorption rate was the most likely reason for the PK difference seen for 2 consecutive visits of the participants.
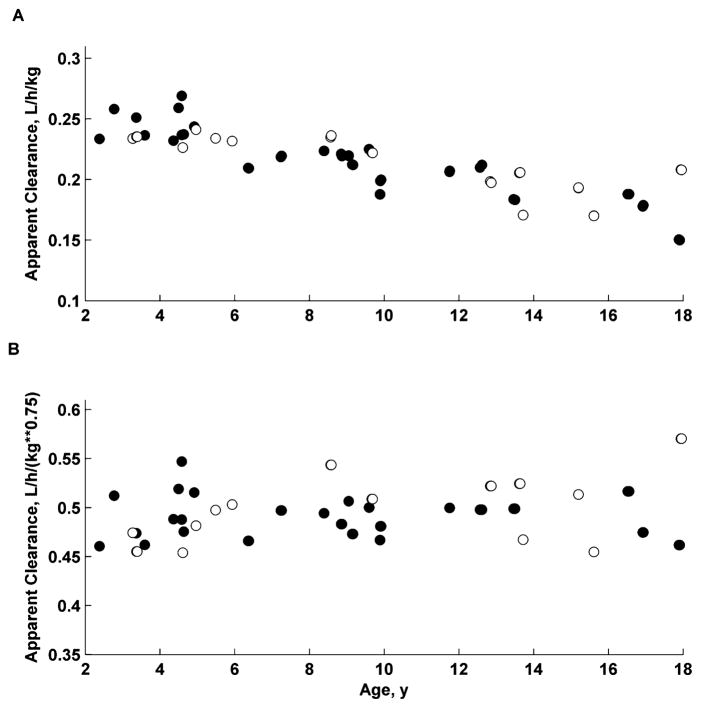
The apparent volume of distribution equaled 45.4 L, and the apparent systemic clearance was 11.9 L/h for a theoretical patient of 70 kg. Allometric scaling accounted for the weight differences observed in disposition parameters (CL/F, V/F) with the standard exponent of 0.75 for clearance and 1 for distribution volume. The IIV for CL/F and V/F were small (CV% less than 10%). The mean absorption time was almost 2-fold greater for capsules (0.318 hours) than for liquid (0.181 hours), with high IOV of 63%. The graphical presentation of weight, sex, and formulation effects on PK parameters is provided in Figure 2.
Figure 2.
Relationship of body-weight-normalized apparent clearances (CL/F/BW and CL/F/BW^0.75) vs age for all children. Closed symbols (●) denote males, and open symbols (○) denote females.
Age, sex, height, and laboratory variables (Table 1) were not significant covariates in this study (data not shown).
Safety Results
All participants tolerated the single dose of hydroxyurea and all study-related procedures without apparent adverse events attributed to them. AE data are provided in Table S4.
Discussion
Hydroxyurea is a crucial therapy for children with SCA. Its off-label designation for children concerns parents and has been identified as a significant barrier to widespread acceptance.20 In addition, only having access to capsule formulations can impede pediatric use both because toddlers and many older children lack the ability to swallow capsules21 and because clinicians have difficulties in adapting fixed capsule formulations to dosing in small children. This trial specifically addresses pharmacologic limitations that had been identified by the FDA as necessary for regulatory approval for pediatric use and liquid formulation: comparative PK data on the bioavailability of liquid vs capsule formulations and the PK profile of HU in children across cohorts of different ages. Many drugs experience age-related differences in absorption, distribution, metabolism, and excretion that have therapeutic implications.22 Based on FDA guidelines,15 our data conclusively address both regulatory limitations. A liquid preparation of HU (100 mg/mL) diluted in cherry syrup is bioequivalent to Droxia® (200 mg) capsules. Furthermore, toddlers who received a liquid formulation of HU had similar PK profiles to older children who received either liquid or capsule formulations. Independent of formulation or age between 2 and 18 years, toddlers and children with SCA had rapid absorption that translated to a predictable disposition of the medication.
Currently, there are limited data evaluating the PK of HU in children. Previous studies have shown that HU has excellent oral bioavailability23 with rapid but variable absorption and rapid elimination with half-life of approximately 2 hours.16,17,24 In this prospective, open-label trial, we investigated the PK in children following ingestion of liquid and/or capsule formulations of HU followed by early and frequent blood sampling. Both formulations had rapid absorption (Tmax 0.86 hours) with a peak concentration of 33.8 μg/mL. Interpersonal variation was observed in the absorption time of HU in both younger and older children. This variation has been reported17 and may be due to variations in transmembrane transporters.25 Between liquid and capsule formulations, the largest difference in the PK profiles was a trend toward a shorter time to peak concentration following ingestion of the liquid compared with the capsule, but that difference did not reach statistical significance (0.74 versus 0.97 hours, P = 0.14; Table 2). Pediatric PK data for HU are limited to 3 studies: observations from the internal pilot study (consisting of the first 22 participants) of the NIH-sponsored BABY HUG trial,16 a study of children with SCA administered an oral tablet formulation,18 and children receiving a first-dose of HU via a liquid suspension17 (Table 4). In the NIH-sponsored BABY HUG trial,16 as part of an internal pilot study, 22 very young children (mean age 14.7 months) underwent PK sampling following their first dose of liquid HU. PK samples were collected predose and then 1, 2, and 4 hours following ingestion. The Cmax and AUC∞ (19.8 μg/mL and 68.8 μg · h/mL) were lower than our results and those of subsequently published pediatric studies.18 At the time, the authors felt this was suggestive that the PK profile of liquid HU in very young children may be different from that in adults. Subsequently, de Montalembert et al18 reported PK profiles in 11 children at a mean (SD) age of 10.2 (5.5) years who ingested a coated breakable HU tablet. The Cmax in that population was 24.5 μg/mL, with an AUC∞ of 115.8 μg · h/mL. Finally, Ware et al17 performed PK testing following a first dose of HU (liquid, n = 77; capsule, n = 10) at mean (SD) age of 9.6 (4.8) years. Children given the liquid formulation had a lower peak concentration (25.4 μg/mL) and shorter half-life (1.64 hours) but a similar AUC∞ (91.9 μg · hr/mL) when compared with children given a capsule (31.9 μg/mL, 2.17 hours, and 101.0 μg · h/mL).
In contrast to previous studies in children, we pursued a protocol of early and aggressive monitoring of plasma concentrations. Liquid and capsule formulations resulted in similar AUC∞, similar Cmax, and similar half-life (Table 2). In our participants, 21.0% (8/39) of children receiving a liquid formulation reached a peak drug concentration between 30 and 60 minutes. The first scheduled blood sampling in the BABY HUG trial’s internal pilot occurred at 1 hour following HU ingestion. This time point was likely after the peak concentration had been reached and during the precipitous phase of drug elimination, which may have potentially overestimated the Tmax and underestimated the Cmax and AUC∞. All 11 children reported by de Montalembert et al reached Tmax at the first blood sample draw, which was 45 minutes following dosing.18 This timing also suggests that participants may have reached Tmax prior to sampling. In contrast, the 77 children reported by Ware et al included early blood sampling (15 and 30 minutes postdose). However, samples were not obtained between 30 minutes and 1 hour,17 which is the time frame that 1 in 5 children given the liquid reached peak drug concentrations in our cohort. That timing may have led to a longer calculated Tmax and underestimated Cmax and AUC∞.
We report that a population PK model was successfully developed to describe the time course of HU concentrations after oral administration in children with SCA. This model adequately captured the variability in absorption and disposition of HU. Body weight was found to influence both the apparent clearance and volume of distribution and was the main factor that varied with age. Measures of medication exposures (AUC- or CL/F-related) were not altered by formulation; therefore, dosing adjustments are not required for transitioning from one formulation to another. Additionally, this model offers confidence, with respect to dosing and PK, that current standard-practice dosing schemes that adjust for weight result in similar drug exposures over time.
One limitation of this study is that we did not ascertain if differences exist in PK parameters following a single dosage and during steady-state sampling with chronic daily administration. Although this was initially planned as an aim of this study, it was unable to be completed due to difficulty in recruiting participants. Also, this study did not assess the PK of HU in infants or in children less than 2 years of age.
In summary, a solution of HU in cherry syrup compared with Droxia® capsules meets FDA requirements of bioequivalence in children.15 Our results demonstrate that (1) exposure to HU is not significantly altered based on liquid or capsule formulation, making dosing adjustments for differences in either drug formulation or age unnecessary, and (2) standard weight-based dosing schemes of HU provide consistent drug exposure. These data support the use of liquid HU in children who are unable to swallow capsules and in those whose low weight precludes the use of fixed capsule formulations. These findings should encourage the use of HU across the spectrum of both age and weight in children with SCA, and they should facilitate the expanded use of HU as recently recommended in the NHLBI guidelines for care of children with SCA. Importantly, satisfying FDA regulatory requirements for pediatric approval of HU could address additional barriers to use.
Supplementary Material
Acknowledgments
This clinical trial was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), under the authority of the Best Pharmaceuticals for Children Act (BPCA), contract no. HHSN201000003I/task order no. HHSN27500004 (K.A.N. and Z.R.).
The authors would like to thank the children and families who participated in the study. The authors also appreciate the efforts of the laboratory personnel, study coordinators, and nursing staff at each of the participating institutions.
The Pediatric Trials Network Administrative Core Committee
Daniel K. Benjamin Jr, Duke Clinical Research Institute, Durham, NC; Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, University of California–San Diego, San Diego, CA; Michael Cohen-Wolkowiez, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Children’s Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andre Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O’Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; P. Brian Smith, Duke Clinical Research Institute, Durham, NC; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Thomas J. Walsh, Weill Cornell Medical College of Cornell University, New York, NY.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Alice Pagan.
The Emmes Corporation (Data Coordinating Center): Ravinder Anand, Gina Simone.
Footnotes
ClinicalTrials.gov identifier: NCT01506544
Author Contributions
J.H.E. enrolled participants, analyzed the results, and wrote the manuscript; C.M. designed and executed the protocol and provided critical review and editing of manuscript; C.D.T. enrolled participants and provided critical review and editing of manuscript; P.W. analyzed results and provided critical review and editing of manuscript; Z.R. contributed to protocol design, enrolled participants, and provided critical review and editing of manuscript; J.A.R., N.S.G., R.L., A.M.B., S.E.C., and T.H.H. enrolled participants and provided critical review and editing of manuscript; M.H.M. designed and executed the protocol and provided critical review and editing of manuscript; A.L. designed the protocol, analyzed results, and provided critical review and editing of manuscript; U.G. designed the protocol, analyzed samples, and provided critical review and editing of manuscript; W.J.J. contributed to the overall research design and protocol design, analyzed results, and critically reviewed and edited the manuscript; K.A.N. contributed to the overall research design and protocol design, served as study chair and IND holder, executed the protocol, analyzed results, and wrote the manuscript.
Conflict-of-Interest Disclosures
Jeremie H. Estepp receives funding support from Daiichi Sankyo and Eli Lilly and Co. Courtney D. Thornburg has research funding from MAST Pharmaceuticals for sickle-cell-related research; she also received NIH funding for the BABY HUG follow-up studies. Zora Rogers has received honoraria and consultation work from Apopharma, Baxter, Roche, and GSK as well as from the Texas Department of State Health Services. Shelley E. Crary has served on the advisory board for hemophilia for Grifols. The other authors have no potential conflicts to report.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site
References
- 1.Brousseau DC, Panepinto A, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–78. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 2.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 3.Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106(7):2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 5.Lopes de, Castro Lobo C, Pinto JFC, Nascimento EM, et al. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161(6):852–860. doi: 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 6.Thornburg C, Dixon N, Burgett S, et al. A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer. 2009;52(5):609–615. doi: 10.1002/pbc.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WC, Wynn LW, Rogers ZR, et al. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139(6):790–796. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- 8.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 10.Yawn BP, Buchanan GR, Afenyi-Annan AN. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 11.McGann PT, Howard TA, Flanagan JM, Lahti JM, Ware RE. Chromosome damage and repair in children with sickle cell anaemia and long-term hydroxycarbamide exposure. Br J Haematol. 2011;154(1):134–140. doi: 10.1111/j.1365-2141.2011.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGann PT, Flanagan JM, Howard TA, et al. Genotoxicity associated with hydroxyurea exposure in infants with sickle cell anemia: results from the BABY-HUG phase III clinical trial. Pediatr Blood Cancer. 2012;59(2):254–257. doi: 10.1002/pbc.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott DK, Neville K, Garg U. Determination of hydroxyurea in serum or plasma using gas chromatography-mass spectrometry (GC-MS) Methods Mol Biol. 2010;603:279–287. doi: 10.1007/978-1-60761-459-3_26. [DOI] [PubMed] [Google Scholar]
- 14.Garg U, Scott D, Frazee C, Kearns G, Neville K. Isotope dilution gas chromatography-mass spectrometry (GC-MS) method for the analysis of hydroxyurea. Ther Drug Monit. 2015;37(3):325–330. doi: 10.1097/FTD.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. [Accessed March 25, 2015];Statistical Approaches to Establishing Bioequivalence. 2001 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070244.pdf.
- 16.Rogers ZR, Thompson B, Ware RE, et al. Pharmacokinetics of hydroxyurea in young children with sickle cell anemia: a report from the BABY HUG trial. Blood (ASH Annual Meeting Abstracts) 2005;106:3184. [Google Scholar]
- 17.Ware RE, Despotovic J, Mortier NA, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood. 2011;118(18):4985–4991. doi: 10.1182/blood-2011-07-364190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Montalembert M, Bachir D, Hulin A, et al. Pharmacokinetics of hydroxyurea 1000 mg coated breakable tablets and 500 mg capsules in pediatric and adult patients with sickle cell disease. Haematologica. 2006;91(12):1685–1688. [PubMed] [Google Scholar]
- 19.Yan JH, Ataga K, Kaul S, et al. The influence of renal function on hydroxyurea pharmacokinetics in adults with sickle cell disease. J Clin Pharmacol. 2005;45(4):434–445. doi: 10.1177/0091270004273526. [DOI] [PubMed] [Google Scholar]
- 20.Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer. 2013;60(4):653–658. doi: 10.1002/pbc.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekele E, Thornburg C, Brandow AM, et al. Do difficulties in swallowing medication impede the use of hydroxyurea in children? Pediatr Blood Cancer. 2014;61(9):1536–1539. doi: 10.1002/pbc.25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez GI, Kuhn JG, Weiss GR, et al. A bioavailability and pharmacokinetic study of oral and intravenous hydroxyurea. Blood. 1998;91(5):1533–1541. [PubMed] [Google Scholar]
- 24.Wiczling P, Liem RI, Panepinto JA, et al. Population pharmacokinetics of hydroxyurea for children and adolescents with sickle cell disease. J Clin Pharmacol. 2014;54(9):1016–1022. doi: 10.1002/jcph.303. [DOI] [PubMed] [Google Scholar]
- 25.Walker AL, Lancaster CS, Finkelstein D, Ware RE, Sparreboom A. Organic anion transporting polypeptide 1B transporters modulate hydroxyurea pharmacokinetics. Am J Physiol Cell Physiol. 2013;305(12):C1223– C1229. doi: 10.1152/ajpcell.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.