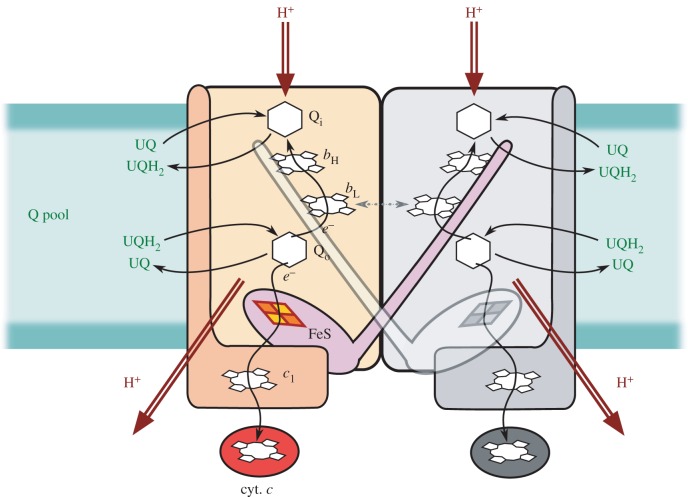
Figure 1.
Diagram of homodimeric cytochrome bc1 structure describing the general mechanism of enzymatic turnover. The ubiquinone binding sites Qi, Qo together with haems bL and bH (b-chain) are embedded in cytochrome b subunit (light orange rectangle). The Rieske protein (light magenta) harbouring 2Fe–2S (FeS) iron–sulfur cluster and cytochrome c1 subunit (dark orange) with haem c1 transfer the electrons from Qo site to cytochrome c (red). The proton uptake and release is indicated by red arrows. The intermonomer electron transfer at the level of two haems bL [5,6] is indicated by dashed arrow. For clarity, the second monomer is shown in grey. Ubiquinone (UQ) and ubiquinol (UQH2) constitute the Q pool.