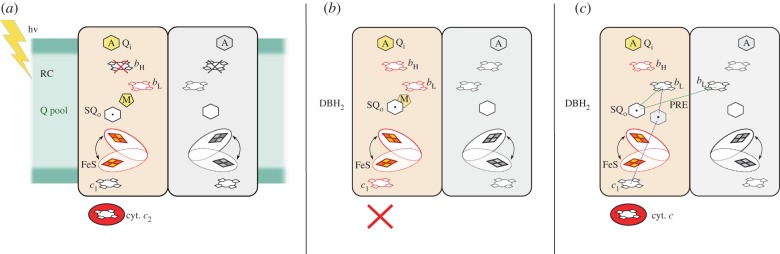
Figure 2.
Schematic of the SQ intermediate trapped in the Qo site with the corresponding enzyme state as reported in (a) [33], (b) [34] and (c) [35]. The redox states of cytochrome bc1 cofactors (FeS and haems) were either reduced or oxidized (red or black contour, respectively). In all cases, the Qi site was occupied by antimycin (A). Myxothiazol (M) did not preclude the SQo trapping in (a). In (b), the authors speculated that SQo is formed in the vicinity of myxothiazol binding site. In (c), dotted green lines denote the possible dipole–dipole interaction of SQo with haems what lead to paramagnetic relaxation enhancement (PRE) of SQo. The analysis of PRE resulted in assigning two possible locations of for SQo within the Qo site. For simplicity, the second cytochrome bc1 monomer was shaded. In (a) the reaction was initiated by light activation of reaction centre (RC), while in (b) and (c) the reaction was initiated by injection of the reduced ubiquinone analogue (DBH2).