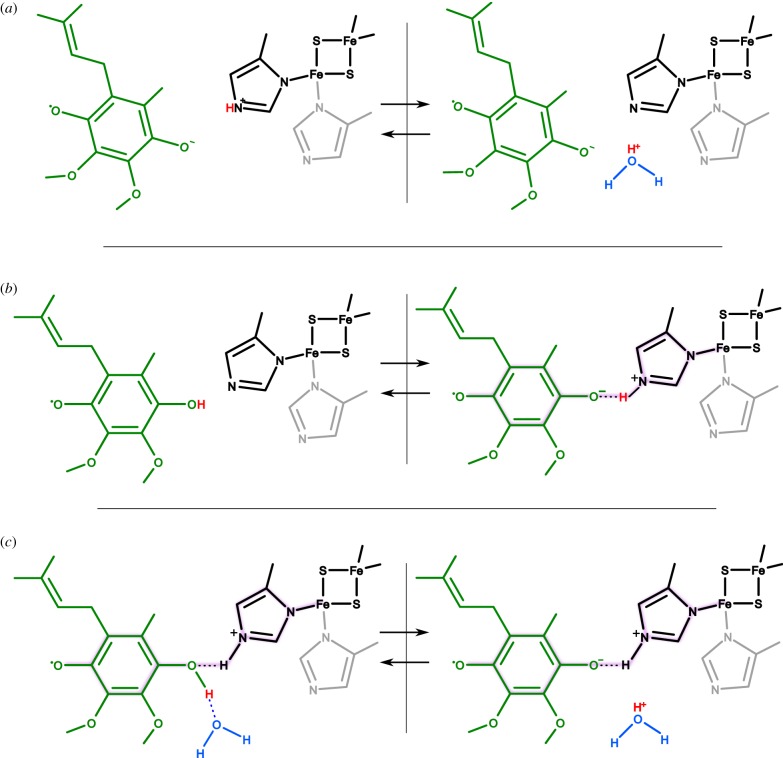
Figure 4.
Different possibilities of proton involvement in the interactions of SQo (green) with the cluster liganding histidine (black) and/or water (blue). (a) SQo anion does not form a hydrogen bond with the histidine that reversibly exchange proton (red) with water molecules (arrows represent the reversibility of the reaction). (b) Protonated SQo reversibly donates proton to histidine which results in formation of hydrogen bond between this histidine and SQo anion. (c) Neutral SQo forms hydrogen bond with protonated histidine, whereas the proton originating from SQo is exchanged with water molecule. All three cases (a–c) may exist in an equilibrium but only in cases (b, right-hand panel) and (c, both panels) is a relatively strong spin–spin exchange interaction expected between SQo and the FeS cluster (magenta shadow shows the possible paths for the electron spin exchange).