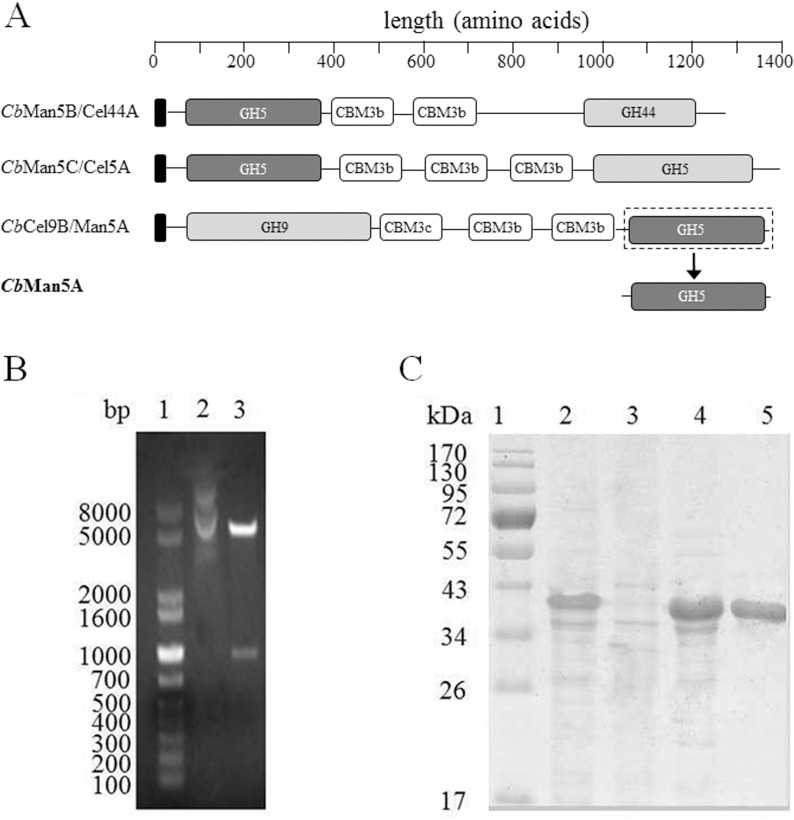
Fig 1. Cloning, expression, and purification of CbMan5A.
A: Domain structure showing the three unique multimodular proteins with a conserved GH5 domain. The conserved GH5 domains are filled with dark grey, the signal peptides are filled with black, and the other domains are filled with white (CBMs) or light grey (catalytic domains). CbMan5B/Cel44A, CbMan5C/Cel5A, and CbCel9B/Man5A are also referred as Athe_1859, Athe_1866, and Athe_1865, respectively. B: Restriction digestion of pET-cbMan5A. Lane 1: DNA molecular mass marker; 2: undigested pET-cbMan5A; 3: pET-cbMan5A digested with EcoRI and NotI. C: SDS-PAGE analysis of purified CbMan5A. Lane 1: protein molecular mass marker; 2: whole cell lysate of pET-cbMan5A/BL21(DE3); 3: cell debris; 4: supernatant; 5: purified CbMan5A.