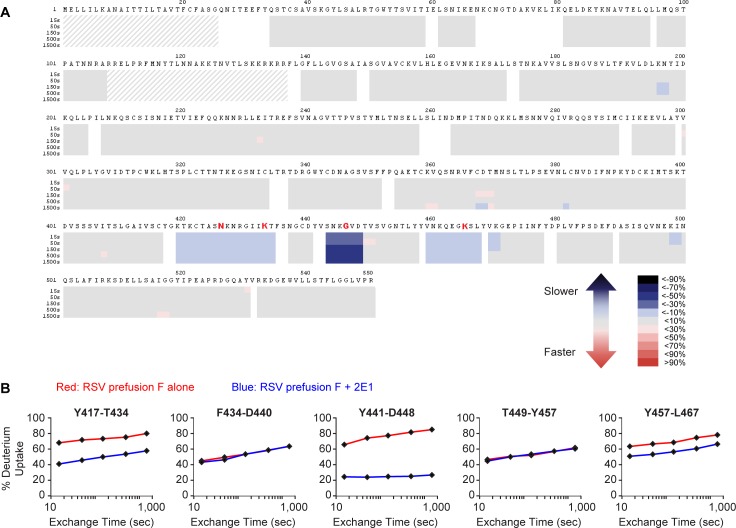
Fig 5. Epitope mapping of 2E1 by hydrogen/deuterium-exchange mass spectrometry.
(A) Heat map plot showing the difference in deuteration levels of the RSV prefusion F protein alone compared to RSV prefusion F protein in the presence of the 2E1 monovalent Fab at five time points (15, 50, 150, 500, and 1500 sec). Slower deuterium exchange indicates regions containing the binding sites. White areas are ‘gaps’ for which there was no sequence coverage, and thus no HDX-MS information was obtained. Dashed greyed-out areas represent sequences of the signal and P27 peptides which are not present in the mature purified protein. (B) Uptake plots of several RSV F peptides spanning the conformational epitope region. Red curves show the deuteration levels of peptides of RSV F protein alone, while blue curves show the peptides of the RSV F / 2E1 complex. Peptides containing the residues of antibody epitope (417–434, 441–448, and 457–467) showed decreased deuteration level upon 2E1 binding. In contrast, peptides with no significant decrease in the deuteration level upon 2E1 exposure represent non-epitope sequences (435–440 and 449–457). The residues identified as critical for binding by shotgun mutagenesis are indicated in red font.