Abstract
Smooth muscle cells (SMCs) are key regulators of vascular disease and circulating smooth muscle progenitor cells may play important roles in vascular repair or remodelling. We developed enhanced protocols to derive smooth muscle progenitors from murine bone marrow and tested whether factors that are increased in atherosclerotic plaques, namely platelet-derived growth factor—BB (PDGF-BB) and monomeric collagen, can influence the smooth muscle specific differentiation, proliferation, and survival of mouse bone marrow-derived progenitor cells. During a 21 day period of culture, bone marrow cells underwent a marked increase in expression of the SMC markers α-SMA (1.93 ± 0.15 vs. 0.0008 ± 0.0003 (ng/ng GAPDH) at 0 d), SM22-α (1.50 ± 0.27 vs. 0.005 ± 0.001 (ng/ng GAPDH) at 0 d) and SM-MHC (0.017 ± 0.004 vs. 0.001 ± 0.001 (ng/ng GAPDH) at 0 d). Bromodeoxyuridine (BrdU) incorporation experiments showed that in early culture, the smooth muscle progenitor subpopulation could be identified by high proliferative rates prior to the expression of smooth muscle specific markers. Culture of fresh bone marrow or smooth muscle progenitor cells with PDGF-BB suppressed the expression of α-SMA and SM22-α, in a rapidly reversible manner requiring PDGF receptor kinase activity. Progenitors cultured on polymerized collagen gels demonstrated expression of SMC markers, rates of proliferation and apoptosis similar to that of cells on tissue culture plastic; in contrast, cells grown on monomeric collagen gels displayed lower SMC marker expression, lower growth rates (319 ± 36 vs. 635 ± 97 cells/mm2), and increased apoptosis (5.3 ± 1.6% vs. 1.0 ± 0.5% (Annexin 5 staining)). Our data shows that the differentiation and survival of smooth muscle progenitors are critically affected by PDGF-BB and as well as the substrate collagen structure.
Introduction
It is becoming recognized that vascular smooth muscle progenitor cells (SMPCs) play important roles in vascular repair, remodelling and disease ([1, 2]). Some controversy remains over the relative contribution of SMPCs arising from the local vascular tissue versus those from the bone marrow [3–5], nonetheless, the application of bone marrow derived SMPCs has been shown to limit and stabilize atherosclerotic plaque development in ApoE-/- mice [2] and limit aneurysm progression in rats [6] and mice [7]. In addition, lesion progression in mice can be limited by systemic CXCL12 injections resulting in the recruitment of circulating SMPCs [8] and human studies demonstrate that circulating SMPC levels are reduced in acute coronary syndromes as compared to stable angina [2]. These studies suggest that bone marrow derived SMPCs may be important therapeutic targets that may be modified to favourably induce appropriate vascular remodelling.
SMCs exert complex effects in vascular disease: in normal arteries, SMCs are thought to be ‘silent’ and are harnessed during disease to remodel arteries in response to injury. With adaptive remodelling resulting in stabilized plaques by synthesizing a thick fibrous cap that protects against rupture. SMCs change from their physiological contractile phenotype to the pathophysiological synthetic phenotype and migrate into the intima where they advance disease progression by importing lipid, stimulating inflammation, producing extracellular matrix, and secreting matrix metalloproteinases [9–14]. The phenotypic alteration of SMCs is critically regulated by the matrix in atherosclerosis, with normal components of the healthy SMC matrix limiting modulation and atherosclerosis-associated degraded matrix proteins promoting phenotypic modulation [15]. Excessive collagen breakdown combined with inadequate synthesis weakens plaques thereby making them prone to rupture [16]. Monomeric collagen (non-fibrillar collagen) has been previously shown to reduce SMC contractility and stimulate inflammatory protein expression such as VCAM-1 and NF-kappaB [15, 17]. The monomeric collagen matrix also stimulates SMC proliferation in contrast to the reduction of cell growth on fibrillar collagen [17, 18]. These effects can be further enhanced when co-stimulated with platelet-derived growth factor (PDGF-BB) which possesses potent mitogenic and inflammatory effects and participates in ECM remodeling and has been shown to promote proliferation and differentiation in adjacent SMCs [19–21]
Establishing the origin of neointimal SMCs is central to understanding the pathogenesis of atherosclerosis however it currently remains elusive. It has been shown that a bone marrow cell population can readily differentiate into SMC-like cells depending on local environmental cues and proliferate and migrate to the neointimal SMC layer in vivo ([22], reviewed in [23, 24]). For example, transplanted bone marrow labelled with eGFP, lacZ, or by sex-mismatching has been shown to result in labelled recipient neointimal SMCs in the mechanical vascular injury, allograft vasculopathy and hyperlipidemia-induced models of atherosclerosis [3, 25–27].
In this study, we isolated murine bone marrow cells and differentiated them towards a smooth muscle lineage. We showed that the viability and capacity to maintain a differentiated phenotype of bone marrow derived SMPCs are strongly influenced by monomeric collagen and PDGF-BB, factors that are increased in atherosclerotic plaques. These results suggest that matrix remodelling and increased levels of PDGF-BB either in the plaque or remodelling vasculature microenvironment may limit SMCs involvement in healing atherosclerotic plaques in vivo.
Materials and Methods
Isolation of smooth muscle progenitor cells from mouse bone marrow
All animal procedures were carried out in accordance with the Canadian Council of Animal Care (CCAC) guidelines. the protocol (#573) was approved by the Animal Care Committee of St.Michael’s Hospital, Toronto. Female FVB/N mice were sacrificed at 6–10 weeks of age and bone marrow was collected from femorae and tibiae by aspiration. Harvested cells were dissociated and seeded into flasks at a density of 4 x 105 cells/mm2 in α-MEM containing 10% FBS, 50 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen)(referred as 10% α-MEM throughout the manuscript). Cells were maintained in culture at 37°C in humidified 5% CO2.
As indicated, rat recombinant PDGF-BB (50 ng/mL, Sigma-Aldrich) was added directly to the culture media, alone or in combination with the receptor tyrosine kinase inhibitor AGL2043 (10 μM, Calbiochem) or a non-inhibitory control compound AG9 (10 μM, Calbiochem).
Primary aortic smooth muscle cells
Primary aortic smooth muscle cells (SMCs) were isolated from an additional group of female FVB/N mice [28] as follows: a 2 cm length of aorta was resected from the mid-thoracic to the infrarenal region and placed in chilled DMEM containing 1% HEPES. Aortas were denuded of endothelium and adventitial layers by gentle scraping, chopped into 1 mm cubes, and incubated in DMEM containing 1% HEPES, 1% penicillin/streptomycin, collagenase type I (1.8 mg/mL), elastase type III (0.3 mg/mL), soybean trypsin-inhibitor type I (0.44 mg/mL), and BSA (2 mg/mL). After mechanical dispersion and incubation (1 h, 37°C), cell suspensions were diluted 25-fold in DMEM containing 10% FBS and antibiotics, centrifuged (652 g, 5 min), and resuspended and collected in the same media.
RNA isolation and quantitative real-time PCR
RNA was extracted from mouse bone marrow and aortic tissue using RNeasy (Qiagen) and cDNA was synthesized (Omniscript RT, Qiagen). The expression of α-SMA, SM22-α, SM-MHC, and GAPDH mRNA were detected by qRT-PCR in triplicate assays using SYBR Green or TaqMan (Applied Biosystems) reactions. Details of the PCR and primer sequences are shown in Table 1. The pre-optimized SMMHC primer set (TaqMan, Mm00443013_m1) was purchased from Applied Biosystems).
Table 1. Primer sequences for SMC gene targets.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| α-SMA | 5’-CGGCTTCGCTGGTGATGA-3’ | 5’-TCCCTCTCTTGCTCTGGGCTT-3’ |
| SM-22-α | 5’-CTCTAATGGCTTTGGGCAGTT-3’ | 5’-TGCAGTTGGCTGTCTGTGAAG-3’ |
| GAPDH | 5’-GCATGGCCTTCCGTGTTC-3’ | 5’-ATGTCATCATACTTGGCAGGCAGGTTTC-3’ |
Immunolabelling and image analysis
Cells were fixed in 4% paraformaldehyde in PBS (w/v, pH 7.4) for 15 min, permeabilized in 0.1% Triton X-100 for 30 min, and blocked in 3% normal goat serum in PBS. Mouse anti-human smooth muscle actin IgG2aκ (1:200 in PBS/1% BSA, Clone 1A4, DAKO); rabbit anti-SM22 alpha antibody (1:1000 for both immunostaining and western blot, Abcam); rabbit anti-smooth muscle myosin heavy chain antibody (1:200, Abcam); mouse anti-vinculin (2 μg IgG1/mL, hVIN-1, Sigma); goat anti-mouse IgG-Alexa 488 (1:400, Molecular Probes) were used for immunostaining. F-actin was labelled with phalloidin-Alexa 546 (Molecular Probes), and nuclei were counterstained with TO-PRO-3 (2 μM, Molecular Probes).
Images were captured using a laser-scanning confocal fluorescence microscope (Bio-Rad Radiance) with a 40X objective (NA 1.3). To score expression of SMC markers, cells from each group were counted in 6 fields (minimum 500 cells in total). Cell morphology was quantified by tracing the outline of the cell as judged by cytoskeletal phalloidin staining and measuring the roundness (a value computed by the software which is proportional to the ratio of the perimeter squared over the radius squared) of the cells, with increasing values representing further deviation from cicular.
BrdU incorporation
BMC-SMPCs were pulse-labelled by addition of BrdU (10 μM, Sigma) to the culture media for 24 h. After 10 d, cells were fixed in 4% paraformaldehyde (10 min, RT), permeabilized in 0.1% Triton X-100 (3 min, RT), and DNA was denatured by incubation in 2 N HCl (20 min, 37°C). After neutralization in borate buffer (0.1 M, pH 8.5), BrdU was detected with mouse monoclonal anti-BrdU IgG1 conjugated to Alexa 488 (2 μg/mL, clone PRB-1, Invitrogen), α-SMA was labelled as described above, and nuclei was counterstained with TO-PRO-3.
Western Blot Analysis
BMCs, SMPCs, or aortic SMCs were lysed in lysis buffer (TBS containing 0.1% SDS, 0.02% NaN3, 100 μg/mL PMSF and 1 μg/mL aprotinin) for 20 min on ice, and cleared by centrifugation (14,000 g, 10 min, 4°C). Protein samples were separated by SDS-PAGE on 4–12% gradient gels and Western blots performed using α-SMA primary antibody (1 μg IgG2aκ/mL, clone 1A4, DAKO) and goat anti-mouse secondary antibody conjugated to horseradish peroxidase (1:2,000, Calbiochem).
Preparation of fibrillar and non-fibrillar collagen coatings
Polymerized collagen fibrils were prepared from stock bovine dermal collagen I solution (Vitrogen, Cohesion Technologies Inc., CA) by dilution to 2.4 mg/mL with 10 X PBS, and the pH adjusted to 7.4. Non-fibrillar collagen solution was prepared by diluting the stock in 0.01 N HCl to a final concentration of 2.4 mg/mL. For matrix coating, collagen was incubated for a minimum of 1 h at 37°C to allow gelation (0.2 mL/cm2) and air-dried overnight. Coated surfaces were soaked in PBS or distilled H2O before use.
Measurement of apoptotic cells by Annexin V and PI co-staining
BM-SMPCs or primary murine aortic SMCs were seeded at an initial density of 2.0 x 104 cells/cm2 on TCPS coated with fibrillar or non-fibrillar collagen. Cells were treated with Annexin V staining solution (Roche) and Hoechst 33342 at 24, 48, or 72 h, (Molecular Probes), and the apoptotic index scored via fluorescence microscopy.
Electron microscopy
BMCs were cultured for 10 d to allow differentiation and then seeded onto glass coverslips coated with non-fibrillar or fibrillar collagen. After 2 d, samples were incubated overnight with (0.1 M Na2HPO3, 0.0675 M NaOH, 1% glutaraldehyde, 3.7% formaldehyde, pH 7.3), postfixed for 30 min with 1% osmiumtetraoxide, and embedded in EPON®. Ultrathin sections (60–80 nm) were cut with an ultramicrotome, contrasted with uranyl acetate and lead citrate, and visualized using a JEOL JEM-1230 TEM transmission electron microscope.
Statistical analysis
All data are presented as means (± SEM), where each n values represent data from an individual animal cell isolate. Multiple comparisons were assessed by ANOVA, followed by Tukey or Dunnett’s post hoc test for significance. When multiple hypotheses were tested simultaneously, significance levels were adjusted by the Bonferroni correction as specified.
Results
Smooth Muscle Differentiation of Bone Marrow Cells
Growth characteristics of early and late cultured bone marrow were compared by plating cells at 2.0 x 104 cells/cm2 at 4 or 17 d after bone marrow isolation, and counting cell density over 3 to 4 d period. Early bone marrow cultures proliferated rapidly in 10% α-MEM, with a doubling time of 1.6 d. By 18 d, the projected population doubling time had lengthened to 5.1 d.
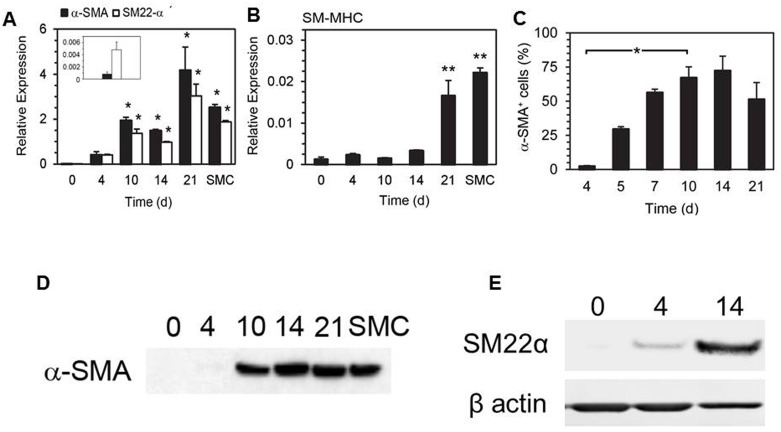
Expression of SMC markers was quantified by qRT-PCR in bone marrow isolates over a 21 day period. In fresh bone marrow, α-SMA and SM22-α were not detected, after 10 d of culture in 10% α-MEM, a significant and sustained increase in expression of both α-SMA and SM22-α was detected (1.93 ± 0.15 fold relative to GAPDH and 1.50 ± 0.27 fold relative to GAPDH, respectively, p < 0.05, ANOVA using Dunnett’s post hoc test) (Fig 1A). Expression of SM-MHC was also significantly increased at 21 d in comparison to fresh bone marrow (0.017 ± 0.004 fold relative to GAPDH vs. 0.001 ± 0.001 fold relative to GAPDH at 0 d, p < 0.01, ANOVA using Dunnett’s post hoc test, n = 3) (Fig 1B). By 21 d, expression of α-SMA, SM22-α and SM-MHC in SMPCs had reached levels comparable to those in cultured murine aortic SMCs. The smooth muscle differentiation was examined at the protein level by Western Blot and Immunocytochemical analysis of α-SMA, SM22-α, and SM-MHC (Figs 1D, 1E and 2). Although α-SMA, SM22-α, and SMMHC were not detected in fresh BMCs, by 14 days their abundance was markedly increased. The percentage of cells expressing cytoskeletal α-SMA underwent a rapid increase during early culture, rising from 2.3 ± 0.5% at 4 d to 67.2 ± 8.0% at 10 d (p < 0.01, Bonferroni multiple comparisons test, n = 3) (Fig 1C).
Fig 1. Bone marrow cells express smooth muscle cell markers upon short-term culture.
qRT-PCR analysis of SMC marker expression in cultured bone marrow cells and murine aortic smooth muscle cells (SMCs) after the indicated culture period. Expression of α-SMA and SM22-α (A) and SM-MHC (B) was normalized relative to GAPDH (* p < 0.05, ** p < 0.01 compared to 0 d, Dunnett’s test, n = 3). The number of α-SMA+ cells was quantified for the indicated time (C). Western blot detection of α-SMA (D) and SM22-α (E) expression of mouse bone marrow cells cultured for the indicated period.
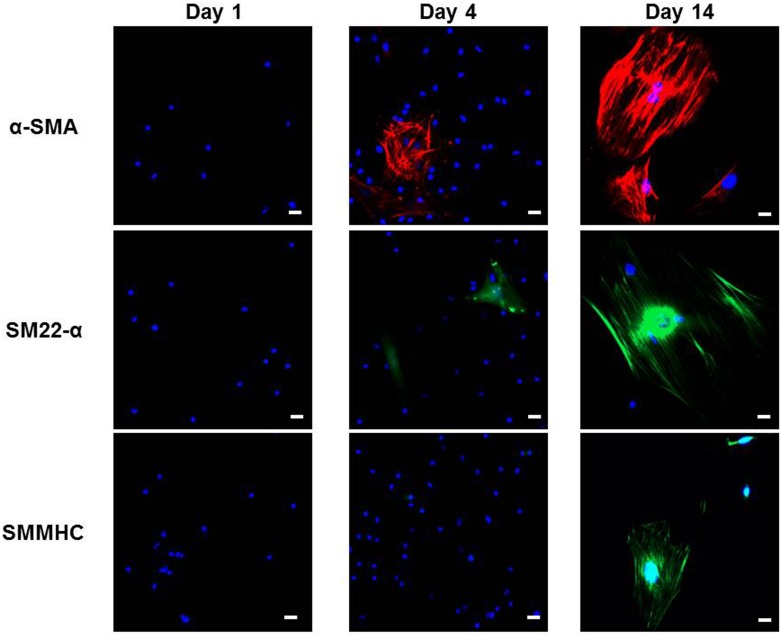
Fig 2. Bone marrow cells express smooth muscle cell markers at different time points.
Immunofluorescence microscopy measurements of α-SMA+, SM22-α+, and SMMHC+ bone marrow cells in culture on days 1, 4, and 14. 20 X magnification, scale bar 20 μm.
Proliferation of α-SMA+and α-SMA- BMCs
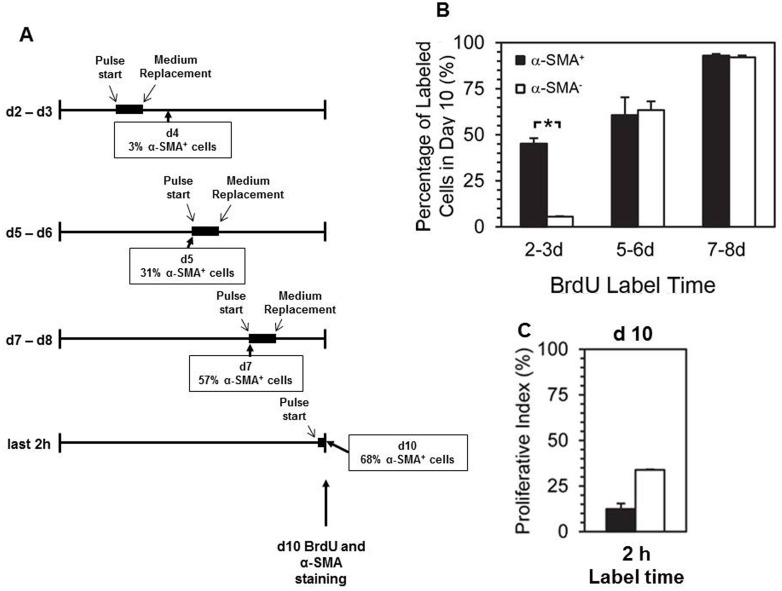
BMCs were pulse-labelled (24 hr) with BrdU at 2, 5, or 7 d and for 2 hours at 10 d after plating, and all cells were immunostained for α-SMA and BrdU at 10 d (Fig 3A). The proportion of cells that were proliferating during each pulse-label was compared between SMPCs (α-SMA+ at 10 d) and other BMCs (α-SMA- at 10 d) (Fig 3B and 3C). Cells committed to SMPC differentiation were highly proliferative in early culture, and their proliferative index increased to 90% by 7 d. α-SMA- BMCs initially displayed very low proliferation (3% BrdU+ cells at 2 d) but this increased at later time points, reaching 60% by 5 d and 90% by 10 d. In early cultures, the difference between proliferative indices in cells differentiating to SMPCs versus not differentiating was highly significant (45.5 ± 5.5% vs. 3.0 ± 0.3% at 2 d, p < 0.01, Bonferroni multiple comparisons test).
Fig 3. Cells committed to SMPC lineage in early bone marrow cultures are marked by a high proliferative index.
BMCs were pulse-labelled with BrdU for a 24 h period starting at days 2, 5, and 7 and subsequently immuno-stained for BrdU and α-SMA at day 10, a subgroup was labelled with BrdU for 2 hours immediately prior to staining, percentages of α-SMA+ cells during culture periods are indicated (A). At day 10 cells were stained for BrdU and α-SMA, the percentage of α-SMA+ and α-SMA- cells labelled with BrdU during the indicated time points are plotted (B and C) (*p < 0.05, Bonferroni multiple comparisons test, n = 3).
SMPC differentiation is modulated by PDGF-BB
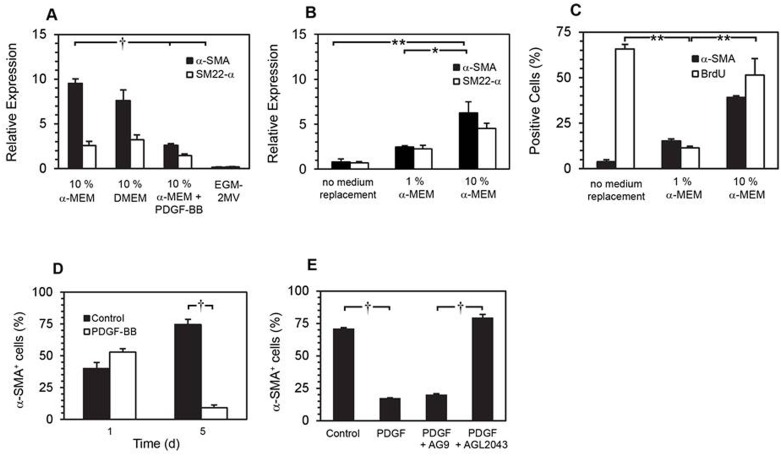
BMCs were cultured in 10% α-MEM, 10% DMEM, 10% α-MEM + PDGF-BB (50 ng/mL) or EGM-2MV for 10 d before qRT-PCR analysis of α-SMA and SM22-α gene expression. At 10 d, robust expression of α-SMA and SM22-α was observed in SMPCs cultured in 10% α-MEM or 10% DMEM, but not in EGM-2MV (an endothelial growth medium used as a negative control). When PDGF-BB (50 ng/mL) was added to 10% α-MEM media, significantly less α-SMA expression was observed (Fig 4A, α-SMA, 3.6-fold less, p < 0.001, Tukey-Kramer multiple comparisons test).
Fig 4. Effects of PDGF-BB on smooth muscle cell marker expression.
qRT-PCR analysis of α-SMA and SM22-α expression in BMCs cultured for 10 d in the specified media (A) († p < 0.001, Tukey-Kramer multiple comparisons test, n = 3). All BMCs were cultured in 10% α-MEM with additional PDGF-BB (50 ng/mL) for 8 d before replacing the media as indicated for 2 d. qRT-PCR analysis of α-SMA and SM22-α expression at 10 d, normalized against GAPDH (B). (* p < 0.05, ** p < 0.01, Bonferroni multiple comparisons test, n = 3). At 10 d, cells were immunolabelled for α-SMA and BrdU (** p < 0.01, Bonferroni multiple comparisons test, n = 3) (C). SMPCs were re-plated at day 10 in 10% α-MEM ± supplemental PDGF-BB (50 ng/mL) for 1 and 5 days, followed by immunofluorescence labelling of α-SMA (D) († p < 0.001, Bonferroni multiple comparisons test, n = 3). SMPC culture media was replaced with: control (no media change), media plus PDGF-BB (50 ng/mL), media plus PDGF-BB (50 ng/mL) and AG9 (10 μM), media plus PDGF-BB (50 ng/mL) and AGL2043 (10 μM), as indicated. Expression of α-SMA was detected by immunofluorescence microscopy after 5 d incubation (E) († p <0.001, Tukey-Kramer multiple comparisons test, n = 3).
In order to test whether suppression of SMC markers by PDGF-BB was reversible, 10% α-MEM + PDGF-BB culture media was replaced at day 8 with α-MEM containing 1 or 10% FBS, and expression of α-SMA and SM22-α was analyzed at day 10. qRT-PCR analysis revealed that α-SMA and SM22-α expression was rapidly restored after PDGF-BB containing media was replaced with 10% α-MEM (Fig 4B). In BMCs cultured in 10% α-MEM + PDGF-BB 10d (control) we observed suppression of α-SMA and high cell proliferation yet replacing the media for 2 d with 10% MEM alone restored α-SMA expression (39.1 ± 0.9%), even though cell proliferation remained high (65.7 ± 2.4% BrdU+), replacing the media with 1% MEM suppressed both α-SMA and cell proliferation (Fig 4C).
The inhibitory effect of PDGF-BB on α-SMA expression was further examined in differentiated SMPCs (10 d culture in 10% α-MEM), no significant change was observed after 24 h treatment, but after 5 d incubation with supplemental PDGF-BB (50 ng/mL) the percentage of α-SMA+ cells was significantly lower (74.5 ± 4.2% vs. 9.2 ± 2.0%, p < 0.001, Bonferroni multiple comparisons test, n = 3, Fig 4D). This α-SMA suppression was receptor dependent as demonstrated by culturing SMPCs in the presence of exogenous PDGF-BB (50 ng/mL) and a PDGFR-selective tyrosine kinase inhibitor (AGL2043, 10 μM) or a negative control compound AG9 (10 μM) for 5 d (79.5 ± 2.5 vs 18.3 ± 0.6%, respectively, p < 0.001, Tukey-Kramer multiple comparisons test, n = 3).
Effects of collagen substrate on SMPC growth and viability
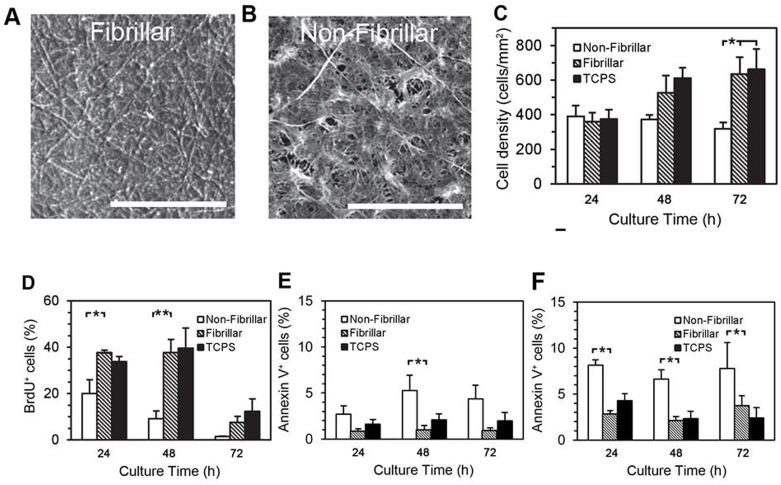
The topography of the collagen coatings was examined by scanning electron microscopy (SEM), and revealed an even coating of collagen fibrils 100 nm wide, reaching lengths upwards of 10 μm in the polymeric (fibrillar) gels (Fig 5A); in contrast the non-fibrillar coatings were distributed in a mesh-like network (Fig 5B). TEM of the acidic collagen solution confirmed the presence of 250 nm by 10 nm rod-like structures, consistent with the expected dimensions for collagen monomers (data not shown). Collagen coating thicknesses of approximately 15 μm were measured on transverse sections by TEM.
Fig 5. Effects of collagen polymerization on SMPC proliferation and viability.
Representative SEM images depicting the topology of surfaces coated with fibrillar (A) or non-fibrillar collagen preparations (B, scale bars are 10 μm). BMCs cultured for 10 d in 10% α-MEM, and then re-plated onto substrates as indicated, at 24, 48 and 72 h, cell density (C) and BrdU incorporation (D) were measured. Indices of Apoptosis were assessed in SMPCs (E) and primary cultures of murine SMCs (F) by Annexin V/PI co-staining. (* p < 0.05, ** p < 0.01, (C, D, n = 4), (E, F, n = 3), Tukey-Kramer multiple comparisons test).
When 10d SMPCs were re-plated onto the collagen gels numbers increased on fibrillar collagen and uncoated TCPS after 48 and 72 h (non-fibrillar vs. fibrillar substrate, 319 ± 36 vs. 635 ± 97 cells/mm2 at 72 h, respectively, p < 0.05, Bonferroni multiple comparisons test, n = 4) (Fig 5C), yet on non-fibrillar collagen SMPC failed to grow over the 72 h period. Pulse-labelling of 10 d SMPCs with BrdU (10 μM, 24 h) confirmed that SMPC proliferation on non-fibrillar collagen was significantly less than that observed on fibrillar collagen or on TCPS (Fig 5D) (non-fibrillar vs. fibrillar collagen, p < 0.05 at 24 h, p < 0.01 at 48 h, Bonferroni multiple comparisons test, n = 4). Annexin V staining demonstrated a small but significant increase in the number of apoptotic SMPCs on non-fibrillar collagen, compared to fibrillar collagen and TCPS (5.3 ± 1.6% vs. 1.0 ± 0.5% at 48 h (fibrillar), p < 0.05, Bonferroni multiple comparisons test, n = 4) (Fig 5E). Similarly, apoptosis was increased in primary aortic SMCs plated on non-fibrillar collagen (Fig 5F).
Impact of collagen substrate on SMPC adhesion and differentiation
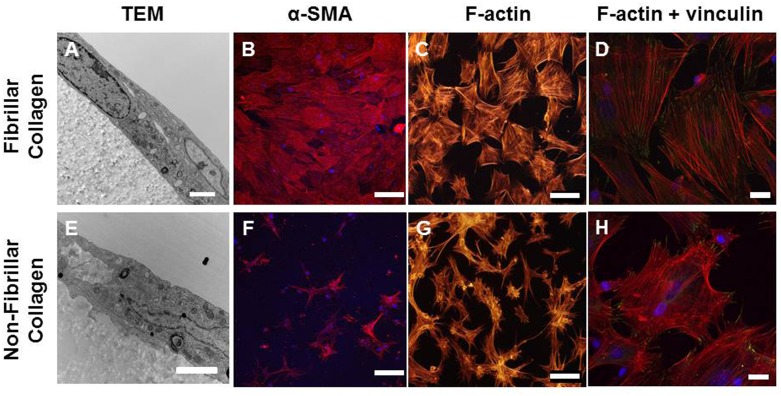
We performed a microscopic comparison of the attachment of SMPCs to fibrillar and non-fibrillar collagen. After 48 h of culture on collagen, TEM images showed uninterrupted contact between the basal membranes of SMPCs and fibrillar collagen-coated surfaces (Fig 6A). On non-fibrillar collagen, however, only discrete attachment points were observed, and were interspersed with gaps up to 500 nm wide between SMPCs and the substrate (Fig 6E).
Fig 6. SMPC differentiation and focal adhesion formation are suppressed on monomeric collagen.
Bone marrow cells were cultured for 10 d in 10% α-MEM media on TCPS to allow expression of SMC markers and re-plated onto fibrillar or non-fibrillar collagen for 72 h. (A, E) TEM images of transverse sections through SMPCs on collagen matrices. Magnification, (A) 10,000 X; (E) 15,000 X. Scale bars 2 μm. (B, F) Representative images for SMA expression at 72 h. (C, G) Phalloidin-Alexa 546 labelling of SMPC F-actin. Magnification, 20 X. Scale bars, 100 μm. (D, H) Co-labelling with phalloidin and mouse anti-vinculin antibody. 60 X magnification, scale bars 20 μm.
The apparently reduced attachment of SMPCs to non-fibrillar collagen was also associated with changes in focal adhesion contacts. SMPC stress fibres and focal adhesions were detected by phalloidin and vinculin co-labelling. After 48 h culture on fibrillar collagen, SMPCs displayed a polygonal morphology with focal adhesion contacts dispersed across the basal surface (Fig 6C and 6D). On non-fibrillar collagen, SMPCs had a stellate appearance and a limited number of focal adhesion contacts were restricted to the periphery of the cell (Fig 6G and 6H). The number of focal adhesion sites per cell was three fold higher in cells cultured on fibrillar as opposed to monomeric collagen.
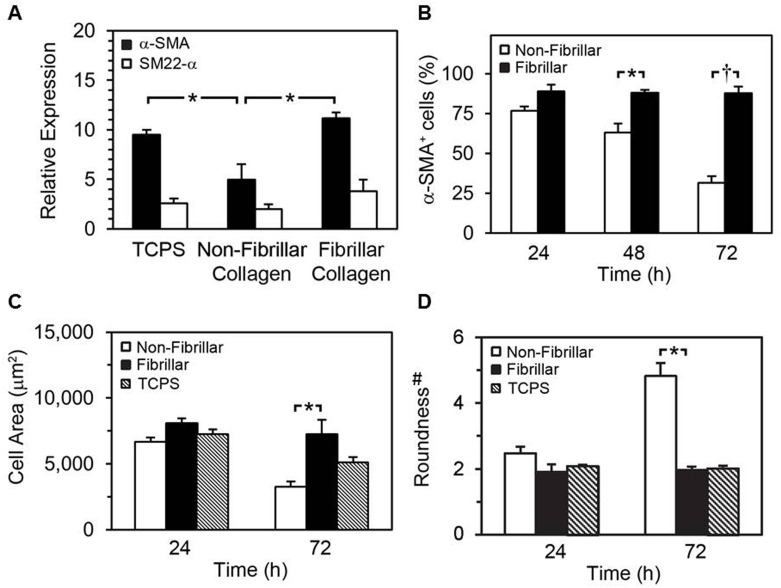
To investigate whether acquisition of the SMPC phenotype was influenced by interactions with substrate collagen, BMCs were cultured on collagen immediately after isolation, and expression of α-SMA and SM22-α was measured by qRT-PCR after 10 d. In line with the trace expression levels of α-SMA and SM22-α previously detected by qRT-PCR in fresh BMCs (Fig 1), expression of both genes was stimulated after 10 d of culture on fibrillar collagen or TCPS (Fig 7A). On non-fibrillar collagen, the stimulatory effect on α-SMA expression was reduced compared to fibrillar collagen (6.1 ± 0.6 compared to 11.2 ± 0.6 fold relative to GAPDH, p < 0.01, Bonferroni multiple comparisons test, n = 3).
Fig 7. Effects of monomeric collagen on SMPC differentiation and cell shape.
qRT-PCR analysis of expression of α-SMA and SM22-α mRNA in BMCs cultured for 10 d on the indicated substrates (A) (* p < 0.01, Bonferroni multiple comparisons test, n = 3). Bone marrow cells were cultured for 10 d in 10% α-MEM media on TCPS followed by re-plating on indicated collagen surfaces for 24 to 72 h. Quantification of α-SMA+ cells at indicated period via immunofluorescence microscopy using mouse anti-αSMA antibody and DAPI (B).(* p < 0.05, † p < 0.001, Bonferroni multiple comparisons test, n = 3). Morphometric analysis of cell area (C) and roundness (D) after culture for 24 and 72 h on collagen was achieved. #: roundness is proportional to the ratio of the perimeter squared over the radius squared of the cells, with increasing values representing further deviation from circular.
Lastly, we measured the effects of the collagen substrate on differentiation in established SMPCs by quantifying the percentage of α-SMA-expressing cells during a 72 h period of culture (Fig 7B). Expression of α-SMA was robust and sustained in SMPCs grown on fibrillar collagen. On non-fibrillar collagen, the number of α-SMA+ cells progressively decreased, reaching 31.6 ± 4.2% by 72 h (vs. 87.7 ± 4.3 for fibrillar collagen, p < 0.001, Bonferroni multiple comparisons test, n = 4). In addition, significant differences in SMPC area and roundness were found upon morphometric image analysis after 72 h culture on collagen, (Fig 7C and 7D).
Discussion
Vascular smooth muscle-like cells have been previously derived from a number of extra-arterial sources including peripheral blood and bone marrow and it has been speculated that these cells may play a role in vascular repair or disease progression (reviewed in [29, 30]). However, the subpopulation of smooth muscle progenitor cells in the bone marrow stroma or the conditions driving smooth muscle differentiation have not been fully described. Kashiwakura et al transfected bone marrow stromal cells (BMSCs) with an SM22α promoter-GFP construct and demonstrated that less than 3% of BMSCs stably expressed SM22α at 10 days of culture and these cells went on to express PDGFβR, α-SMA, calponin, and SM-MHC at later time points [31]. Metharom et al have demonstrated that the CX3CR1-expressing cells in murine bone marrow (representing approximately 10% of the cell population) contain the smooth muscle progenitor population and it is these CX3CR1-expressing cells that are involved in the response to vascular injury [27, 32]. In agreement with these previous studies, we found a population of bone marrow cells with a propensity for differentiation towards the smooth muscle cell phenotype; however, unlike Kashiwakura et al we found expression of α-SMA was markedly induced in 67 ± 1% of the cells within 10 days [31]. By 14 days α-SMA, SM22- α, and SM-MHC were all detectable by immunohistochemistry and by 21 days the mRNA expression of SM-MHC was robust, suggesting that cells reach mature SMC phenotype. In our cultures the differentiated SMCs were largely derived from a subset of cells with a high rate of proliferation between day 2 and day 3 after bone marrow plating, suggesting that SMC progenitors can be identified early (by proliferation) and amplified before the appearance of smooth muscle markers (3% α-SMA on day 4). This early proliferation of smooth muscle progenitors may be distinct to our culture conditions and in part explain the more rapid appearance of the smooth muscle cell phenotype. Serum contains a number of components that may, at high concentrations, act as inhibitors for smooth muscle cell growth [33–35] in our study, we used 10% FBS which is much lower than 25% used by Kashiwakura et al [31] and as such may be more favourable for early SMC progenitor proliferation.
PDGF-BB, a potent mitogenic factor in SMCs, is a component in serum (PDGF-BB levels in human serum range from 2.19–10.67 ng/mL, [36, 37]) and has been shown to suppress expression of α-SMA, SM-MHC and SM α-tropomysin in mature SMCs [19, 20, 38]. In our hands supplementing the cultures with additional PDGF markedly suppressed expression of several key markers of smooth muscle cell differentiation, this effect was reversed within 2 days of PDGF withdrawal and was specifically blocked by the PDGFR tyrosine kinase inhibitor AGL2043. The suppression of α-SMA by PDGF was not linked to enhanced cellular proliferation as proliferation rates were similar in cells showing high α-SMA expression supplemented with 10% FBS. It is possible that the high levels of PDGF simply repress expression of key SMC markers and not complete differentiation to the SMC phenotype. The repression of α-SMA by PDGF-BB is consistent with the findings of Dandre et al [39] for sub-confluent cultures of arterial derived smooth muscle cells. Similar to our studies Banai et al used the selective PDGFRβ inhibitor AG1295 on carotid out growth smooth muscle cells showing 5 days of treatment markedly enhanced levels of α-SMA [40]. Regulation of cell differentiation and marker expression may depend on the proportional activation of the PDGF receptors alpha or beta, which differentially regulate RhoE and Rho-associated kinase (ROCK) activity and subsequently cytoskeletal α-SMA depolymerisation [41].
Fibrillar collagen is a major component of the vascular extracellular matrix yet in pathological conditions, excessive degradation and or synthesis of collagen can lead to the accumulation of non-fibrillar forms (reviewed in [42]). Non-fibrillar collagen has been previously shown to reduce SMC contractility, stimulate inflammatory protein expression such as VCAM-1 and NF-kappaB and inhibit SMC differentiation [15, 19]. In this study we produced two collagen substrates by either allowing bovine dermal collagen to polymerize at neutral pH or gelling the collagen under acidic conditions, by this method we produced a substrate with a detectable fibre structure or one principally deplete of fibres (Fig 5A and 5B and S1 Fig). We observed that the proliferation of SMPCs was markedly reduced and apoptosis increased on non-fibrillar compared to fibrillar collagen. In contrast Koyama et al demonstrated that polymerized fibrillar collagen arrests adult smooth muscle cell proliferation while monomeric collagen supports cell division[17]. These opposing results may be due to the differences in cell types, SMPCs versus adult SMCs in the two studies, or more likely due to differences in the collagen substrates. In our study we used gels with a defined thickness as opposed to the thin coatings employed by Koyama et al, when we used similar thin coatings we also observed a significant decrease of proliferation in SMPCs on fibillar collagen compared to non-fibrillar collagen (data not shown). Hydroxyproline assays used to quantify collagen content after coating demonstrated Koyama’s method (specifically for non-fibrillar collagen) resulted in little protein deposition (S1 Fig) suggesting that the cell response to these very thin coatings are likely influenced by the rigid plastic substrate as has been demonstrated for hMSCs [43]. Rigid substrates support enhanced smooth muscle cell proliferation [44] and differentiation [45] from bone marrow-derived MSCs compare to soft surface which is similar to the response to non-fibrillar collagen seen in Koyama’s study. We believe that the thicker collagen gels more closely represent the three-dimensional scaffold present in the vascular wall.
Differentiation of the bone marrow cells to a smooth muscle phenotype was markedly reduced when the cells were initially plated on non-fibrillar collagen, although this result may also reflect an increase loss of smooth muscle committed cells on this substrate as apoptosis of differentiated SMPCs was higher on non-fibrillar collagen. In agreement with a previous study [19] on smooth muscle cells, we observed that the expression of α-SMA in SMPCs was rapidly suppressed when the cells were re-plated on non-fibrillar collagen yet fibrillar collagen supported the maintenance of SMC marker expression. These shorter term passage studies may better reflect the response to the defined substrate as it is our experience that matrix deposition by SMCs is very limited over a 72 hour time period (S2B and S2C Fig), nor is it likely that substantial matrix is transferred by the cells after dissociation with trypsin [46]. Thus fibrillar collagen substrates appear to be much more favourable to SMPC survival and differentiation and collagen structural changes may play a key role in the vascular response to injury.
In studies with human aortic SMCs Guildford et al demonstrated a maintenance of contractile phenotype with cells grown on a collagen rich matrix and exposed to PDGF [47], whereas Chen et al demonstrated that human aortic SMCs grown on polymerized collagen show high levels of contractile markers (α-SMA, myosin heavy chain) and that expression of these markers is suppressed by the combination of PDGF-BB and IL-1β [19]. In contrast the murine SMPCs and adult aortic SMCs cells used in our studies were responsive to PDGF-BB alone and this may reflect differences in intracellular signalling between the human and murine cell types. A later study by the Chen group has demonstrated the marked utility of polymerized collagen in the derivation of SMCs from human placental-derived multi-potent cells confirming a critical role for the p38 MAPK pathway and serum response factor in matrix induced differentiation and maintenance of SMC phenotype [48]. Taken together these results suggest a subtle balance between the positive effects of a fibrillar matrix and those of PDGF stimulation in the maintenance of a regenerative SMC phenotype in the vascular wall.
Our results confirm that smooth muscle-like cells may be derived from a bone marrow origin, and highlight the critical importance of their interactions with the surrounding matrix. The high levels of PDGF-BB and monomeric collagen, associated with some atherosclerotic plaques, may restrict the differentiation and subsequent survival of SMPCs in the vessel wall. The role of SMPCs in vascular disease has not been fully defined yet appropriate healing in response to vascular injury may be enhanced by therapies designed to enhance SMPC differentiation and survival at the site of injury. In this context, SMPC response to PDGF-BB and collagen structure, in particular, are promising targets for further study.
Supporting Information
TEM images of fibrillar collagen (A). D banding structure was indicated by black arrows. Scale bar indicates 500 nm. The average length of indicated D banding is 18.5 ± 0.2 nm. The collagen content of different coatings was assessed by quantifying their hydroxyproline content. Different methods of collagen coating were indicated. For each type of coating, three preparations were assessed: A) no rehydration, B) rehydration, and C) rehydration with 24 hours cell growth.
(TIF)
Cells were plated on non-fibrillar or fibrillar collagen coated surfaces with or without PDGF-BB (50ng/mL) treatment. At days 1 and 5, percentage of α-SMA positive cells was determined in each culture (A). n = 3. ** and *** indicate p<0.01 and p<0.001, respectively. HASMCs were cultured on TCPS for 72 hours followed by staining with vinculin antibody and phalloidin (B) or decellularization and staining with collagen I antibody (C). Scale bar indicates 20 μm.
(TIF)
Acknowledgments
We are grateful to Ontario Graduate Scholarship for financial support to Y.Y. and NSERC for financial support to C.L.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We are grateful to Ontario Graduate Scholarship for financial support to Y.Y. and NSERC for financial support to C.L.
References
- 1.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108(3):365–77. Epub 2011/02/05. 10.1161/CIRCRESAHA.110.223800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoll J, Fontaine V, Gourdy P, Barateau V, Vilar J, Leroyer A, et al. Role of human smooth muscle cell progenitors in atherosclerotic plaque development and composition. Cardiovasc Res. 2008;77(3):471–80. Epub 2007/11/17. 10.1093/cvr/cvm034 . [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Sata M, Natori T, Kim-Kaneyama JR, Nose K, Shibanuma M, et al. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. FASEB J. 2008;22(2):428–36. Epub 2007/09/13. 10.1096/fj.06-6884com . [DOI] [PubMed] [Google Scholar]
- 4.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26(12):2696–702. Epub 2006/09/30. 10.1161/01.ATV.0000247243.48542.9d . [DOI] [PubMed] [Google Scholar]
- 5.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation. 2007;116(18):2053–61. Epub 2007/10/17. 10.1161/CIRCULATIONAHA.107.722355 . [DOI] [PubMed] [Google Scholar]
- 6.Park HS, Choi GH, Hahn S, Yoo YS, Lee JY, Lee T. Potential role of vascular smooth muscle cell-like progenitor cell therapy in the suppression of experimental abdominal aortic aneurysms. Biochem Biophys Res Commun. 2013;431(2):326–31. Epub 2013/01/08. 10.1016/j.bbrc.2012.12.099 . [DOI] [PubMed] [Google Scholar]
- 7.Zou S, Ren P, Zhang L, Azares AR, Zhang S, Coselli JS, et al. AKT2 Promotes Bone Marrow Cell-Mediated Aortic Protection in Mice. Ann Thorac Surg. 2016. Epub 2016/04/20. 10.1016/j.athoracsur.2016.01.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhtar S, Gremse F, Kiessling F, Weber C, Schober A. CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33(4):679–86. Epub 2013/02/09. 10.1161/ATVBAHA.112.301162 . [DOI] [PubMed] [Google Scholar]
- 9.Pyle AL, Young PP. Atheromas feel the pressure: biomechanical stress and atherosclerosis. Am J Pathol. 2010;177(1):4–9. Epub 2010/06/19. S0002-9440(10)60056-9 [pii] 10.2353/ajpath.2010.090615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–9. Epub 2008/02/16. ATVBAHA.107.159327 [pii] 10.1161/ATVBAHA.107.159327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12(9):1075–80. Epub 2006/08/08. 10.1038/nm1459 . [DOI] [PubMed] [Google Scholar]
- 12.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102(12):1529–38. Epub 2008/05/24. 10.1161/CIRCRESAHA.108.175976 . [DOI] [PubMed] [Google Scholar]
- 13.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. Epub 2004/07/23. 10.1152/physrev.00041.2003 . [DOI] [PubMed] [Google Scholar]
- 14.Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007;39(2):86–93. Epub 2007/10/16. . [PubMed] [Google Scholar]
- 15.Orr AW, Lee MY, Lemmon JA, Yurdagul A Jr., Gomez MF, Bortz PD, et al. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29(2):225–31. Epub 2008/11/22. 10.1161/ATVBAHA.108.178749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41(2):376–84. Epub 1999/05/26. . [DOI] [PubMed] [Google Scholar]
- 17.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87(6):1069–78. Epub 1996/12/13. . [DOI] [PubMed] [Google Scholar]
- 18.Fassett J, Tobolt D, Hansen LK. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006;17(1):345–56. Epub 2005/10/28. 10.1091/mbc.E05-09-0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CN, Li YS, Yeh YT, Lee PL, Usami S, Chien S, et al. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103(8):2665–70. Epub 2006/02/16. 10.1073/pnas.0510973103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Lv W, Piao H, Chu X, Wang H. Role of platelet-derived growth factor-BB (PDGF-BB) in human pulmonary artery smooth muscle cell proliferation. J Recept Signal Transduct Res. 2014;34(4):254–60. Epub 2014/05/09. 10.3109/10799893.2014.908915 . [DOI] [PubMed] [Google Scholar]
- 21.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15(4):197–204. Epub 2004/06/23. 10.1016/j.cytogfr.2004.03.007 . [DOI] [PubMed] [Google Scholar]
- 22.Kruzliak P, Hare DL, Sabaka P, Delev D, Gaspar L, Rodrigo L, et al. Evidence for CD34/SMA positive cells in the left main coronary artery in atherogenesis. Acta Histochem. 2016. Epub 2016/04/19. 10.1016/j.acthis.2016.04.005 . [DOI] [PubMed] [Google Scholar]
- 23.Daniel JM, Sedding DG. Circulating smooth muscle progenitor cells in arterial remodeling. J Mol Cell Cardiol. 2011;50(2):273–9. Epub 2010/11/05. S0022-2828(10)00428-1 [pii] 10.1016/j.yjmcc.2010.10.030 . [DOI] [PubMed] [Google Scholar]
- 24.Bentzon JF, Falk E. Circulating smooth muscle progenitor cells in atherosclerosis and plaque rupture: current perspective and methods of analysis. Vascul Pharmacol. 2010;52(1–2):11–20. Epub 2009/12/03. S1537-1891(09)00150-5 [pii] 10.1016/j.vph.2009.11.005 . [DOI] [PubMed] [Google Scholar]
- 25.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8(4):403–9. . [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93(8):783–90. Epub 2003/09/23. 10.1161/01.RES.0000096651.13001.B4 . [DOI] [PubMed] [Google Scholar]
- 27.Kumar AH, Metharom P, Schmeckpeper J, Weiss S, Martin K, Caplice NM. Bone marrow-derived CX3CR1 progenitors contribute to neointimal smooth muscle cells via fractalkine CX3CR1 interaction. FASEB J. 2010;24(1):81–92. Epub 2009/09/12. 10.1096/fj.09-132225 . [DOI] [PubMed] [Google Scholar]
- 28.Campbell JH, Kocher O, Skalli O, Gabbiani G, Campbell GR. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989;9(5):633–43. Epub 1989/09/01. . [DOI] [PubMed] [Google Scholar]
- 29.Merkulova-Rainon T, Broqueres-You D, Kubis N, Silvestre JS, Levy BI. Towards the therapeutic use of vascular smooth muscle progenitor cells. Cardiovasc Res. 2012. Epub 2012/02/23. cvs097 [pii] 10.1093/cvr/cvs097 . [DOI] [PubMed] [Google Scholar]
- 30.Orlandi A, Bennett M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem Pharmacol. 2010;79(12):1706–13. Epub 2010/02/02. S0006-2952(10)00065-1 [pii] 10.1016/j.bcp.2010.01.027 . [DOI] [PubMed] [Google Scholar]
- 31.Kashiwakura Y, Katoh Y, Tamayose K, Konishi H, Takaya N, Yuhara S, et al. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22alpha promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation. 2003;107(16):2078–81. Epub 2003/04/23. 10.1161/01.CIR.0000070082.64414.B5 . [DOI] [PubMed] [Google Scholar]
- 32.Metharom P, Kumar AH, Weiss S, Caplice NM. A specific subset of mouse bone marrow cells has smooth muscle cell differentiation capacity-brief report. Arterioscler Thromb Vasc Biol. 2010;30(3):533–5. Epub 2009/12/17. ATVBAHA.109.200097 [pii] 10.1161/ATVBAHA.109.200097 . [DOI] [PubMed] [Google Scholar]
- 33.Kubota K, Okazaki J, Louie O, Kent KC, Liu B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J Surg Res. 2003;109(1):43–50. Epub 2003/02/20. . [DOI] [PubMed] [Google Scholar]
- 34.Dorafshar AH, Angle N, Bryer-Ash M, Huang D, Farooq MM, Gelabert HA, et al. Vascular endothelial growth factor inhibits mitogen-induced vascular smooth muscle cell proliferation. J Surg Res. 2003;114(2):179–86. Epub 2003/10/16. . [DOI] [PubMed] [Google Scholar]
- 35.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27(1):25–33. Epub 2012/09/14. 10.1096/fj.12-213744 . [DOI] [PubMed] [Google Scholar]
- 36.Josh F, Tobita M, Tanaka R, Orbay H, Ogata K, Suzuki K, et al. Concentration of PDGF-AB, BB and TGF-beta1 as valuable human serum parameters in adipose-derived stem cell proliferation. J Nippon Med Sch. 2013;80(2):140–7. Epub 2013/05/10. . [DOI] [PubMed] [Google Scholar]
- 37.Solchaga LA, Daniels T, Roach S, Beasley W, Snel LB. Effect of implantation of Augment((R)) Bone Graft on serum concentrations of platelet-derived growth factors: a pharmacokinetic study. Clin Drug Investig. 2013;33(2):143–9. Epub 2013/01/22. 10.1007/s40261-013-0053-5 . [DOI] [PubMed] [Google Scholar]
- 38.Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2013;451(3):375–88. Epub 2013/02/21. 10.1042/BJ20121344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandre F, Owens GK. Platelet-derived growth factor-BB and Ets-1 transcription factor negatively regulate transcription of multiple smooth muscle cell differentiation marker genes. Am J Physiol Heart Circ Physiol. 2004;286(6):H2042–51. Epub 2004/01/31. 10.1152/ajpheart.00625.2003 . [DOI] [PubMed] [Google Scholar]
- 40.Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97(19):1960–9. Epub 1998/06/03. . [DOI] [PubMed] [Google Scholar]
- 41.Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptor-alpha is a key determinant of smooth muscle alpha-actin filaments in bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2007;39(2):379–91. Epub 2006/10/31. 10.1016/j.biocel.2006.09.005 . [DOI] [PubMed] [Google Scholar]
- 42.Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14(1):73–89. Epub 2009/01/16. 10.1177/1358863X08094801 . [DOI] [PubMed] [Google Scholar]
- 43.Leong WS, Tay CY, Yu H, Li A, Wu SC, Duc DH, et al. Thickness sensing of hMSCs on collagen gel directs stem cell fate. Biochem Biophys Res Commun. 2010;401(2):287–92. Epub 2010/09/21. 10.1016/j.bbrc.2010.09.052 . [DOI] [PubMed] [Google Scholar]
- 44.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J Cell Physiol. 2010;225(1):115–22. Epub 2010/07/22. 10.1002/jcp.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32(16):3921–30. Epub 2011/03/15. 10.1016/j.biomaterials.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J Biomed Mater Res A. 2005;75(1):1–13. Epub 2005/08/09. 10.1002/jbm.a.30297 . [DOI] [PubMed] [Google Scholar]
- 47.Guildford AL, Stewart HJ, Morris C, Santin M. Substrate-induced phenotypic switches of human smooth muscle cells: an in vitro study of in-stent restenosis activation pathways. J R Soc Interface. 2011;8(58):641–9. Epub 2010/11/26. 10.1098/rsif.2010.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou MT, Chang SN, Ke C, Chang HI, Sung ML, Kuo HC, et al. The proliferation and differentiation of placental-derived multipotent cells into smooth muscle cells on fibrillar collagen. Biomaterials. 2010;31(15):4367–75. Epub 2010/03/05. 10.1016/j.biomaterials.2010.02.011 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEM images of fibrillar collagen (A). D banding structure was indicated by black arrows. Scale bar indicates 500 nm. The average length of indicated D banding is 18.5 ± 0.2 nm. The collagen content of different coatings was assessed by quantifying their hydroxyproline content. Different methods of collagen coating were indicated. For each type of coating, three preparations were assessed: A) no rehydration, B) rehydration, and C) rehydration with 24 hours cell growth.
(TIF)
Cells were plated on non-fibrillar or fibrillar collagen coated surfaces with or without PDGF-BB (50ng/mL) treatment. At days 1 and 5, percentage of α-SMA positive cells was determined in each culture (A). n = 3. ** and *** indicate p<0.01 and p<0.001, respectively. HASMCs were cultured on TCPS for 72 hours followed by staining with vinculin antibody and phalloidin (B) or decellularization and staining with collagen I antibody (C). Scale bar indicates 20 μm.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.