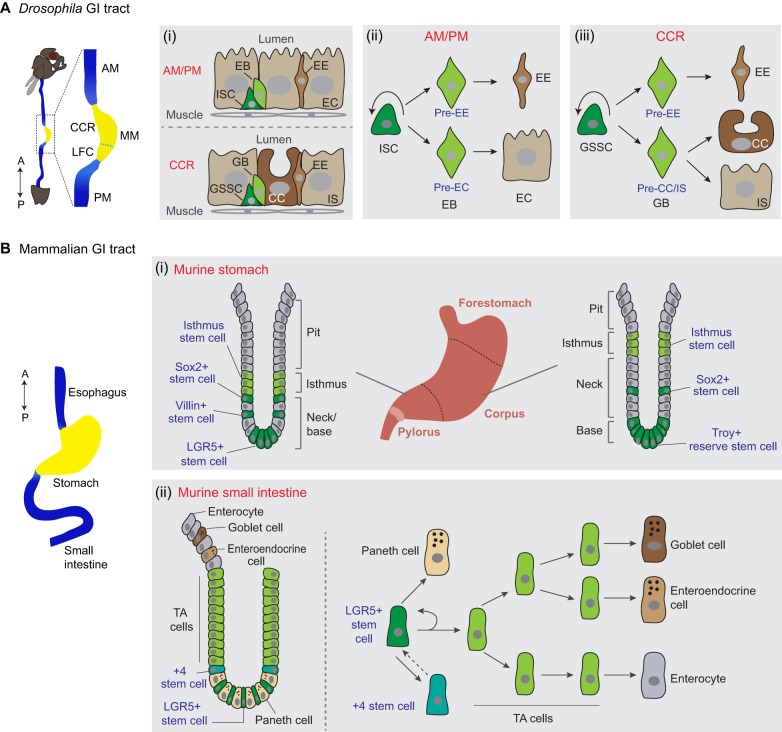
Fig. 1.
Drosophila and mammalian gastrointestinal (GI) tracts and associated stem cell lineages. (A) A schematic of the Drosophila GI tract [‘A’, anterior, top; ‘P’, posterior, bottom), focusing on the midgut, which is divided into three main regions: the anterior midgut (AM), the middle midgut (MM) and the posterior midgut (PM). The MM contains an acidic stomach-like copper cell region (CCR) and a large flat cell region (LFC). (i) Organization of epithelial cells in the AM and PM (top), and the CCR (bottom). (ii) In the AM or PM, intestinal stem cells (ISCs) divide asymmetrically, giving rise to a new ISC and either a pre-enterocyte (pre-EC) enteroblast (EB), which will differentiate into an enterocyte (EC), or a pre-enteroendocrine-cell (pre-EE) EB, which will differentiate into an enteroendocrine cell (EE). (iii) In the CCR, gastric stem cells (GSSCs) undergo similar asymmetric division, giving rise to a new GSSC and two types of gastroblast (GB), which will differentiate into either a copper cell (CC), an interstitial cell (IS) or an EE. (B) The mammalian GI tract consists of an esophagus, stomach, small intestine and large intestine (not shown here) (anterior, top; posterior, bottom). (i) The murine stomach divides into three regions: forestomach, corpus and pylorus. The architecture of the gland unit, including the crypt, in the stomach corpus (right) and pylorus (left) is shown. Different stem cell populations (blue text) have been characterized, but exact stem cell lineages and cell hierarchies are unclear in these regions. (ii) In the murine small intestine, there are two main stem cell populations: +4 stem cells and LGR5+ stem cells, which can replace each other under certain conditions. A schematic of the crypt is shown on the left, and cell lineages are shown on the right. TA cells, transit amplifying cells.