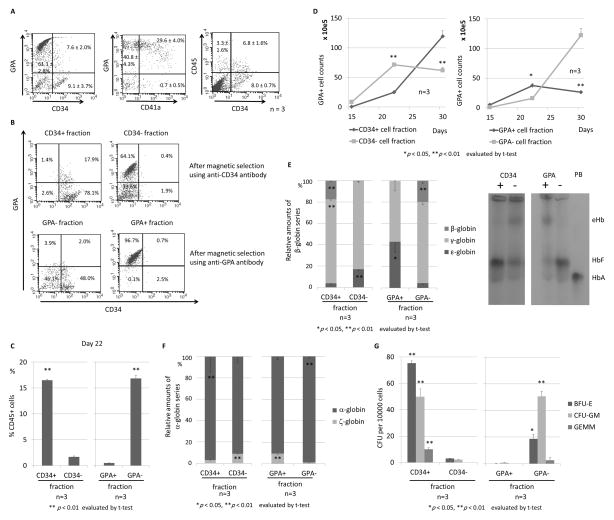
Figure 3. CD34 or GPA selection to distinguish between primitive and definitive erythroid progenitors in ES sacs.
(A). We hypothesized that primitive and definitive erythroid progenitors can be separated by a CD34 or GPA surface marker in ES sacs before erythroid differentiation. We analyzed spherical cells from 15-day ES sacs, in which we observed mainly two populations of GPA+(CD34−) erythroid cells and CD34+(GPA−) HPCs. (B). We separated spherical cells using CD34 or GPA magnetic beads, and obtained a high purity of the CD34+ or − cell fractions as well as the GPA+ or − cell fractions, which were differentiated into erythroid cells. (C). After 5-day erythroid differentiation (day 22), a higher percentage of CD45+ cells was observed in CD34+ or GPA− cell fractions than CD34− or GPA+ cell fractions. (D). During erythroid differentiation (days 17–30), GPA+ erythroid cells efficiently increased in the CD34− or GPA+ cell fractions until day 22 and decreased on day 30, while the erythroid cells slowly increased in the CD34+ or GPA− cell fractions until day 22 and robustly increased on day 30. (E). After 13-day erythroid differentiation (day 30), we observed higher β-globin expression (p < 0.01) and lower ε-globin expression (p < 0.05) in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction. We confirmed the same trend using hemoglobin electrophoresis. (F). After erythroid differentiation (day 30), higher α-globin expression and lower ζ-globin expression were observed in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction. (G). In CFU assay, greater CFUs were observed in the CD34+ or GPA− cell fraction. PB, peripheral blood (expressing HbA); eHb, embryonic hemoglobin; HbF, fetal hemoglobin, HbA, adult hemoglobin.